Pan Wu a, Dongyue Zong b, Lei Yang c, Xuewei Jia a, Lili Qu a, Yihong Wu a, Chunping Xu a
Abstract
Blue lotus (Nymphaea nouchali var. caerulea) is a highly aromatic plant, as well as its processed products, including concrete and essential oil feature distinct aromatic profiles. In this study, the volatile compositions, aroma profiles, and mechanisms underlying aroma perception of three blue lotus products—concrete (NCC), essential oil (NCEO), and hydrosol (NCH)—were systematically investigated. Headspace solid-phase microextraction combined with gas chromatography-mass spectrometry (HS-SPME-GC-MS) identified 81 volatile compounds. Principal component analysis (PCA) revealed that volatile compounds of NCC retained the closest similarity to the fresh flower. A total of 22 key aroma compounds (OAV >1) were identified using OAV calculation, aroma recombination, and omission experiments. Flavoromics approach further highlighted 9 differential compounds (VIP >1, OAV >1, P < 0.05) responsible for aroma attribute difference. All blue lotus processed products prominently featured woody and floral attributes. Molecular docking demonstrated that β-ionone, trans-α-bergamotene, as well as related compounds were capable of binding spontaneously to human olfactory receptors through hydrophobic interactions and hydrogen bonds, contributing to aroma perception. These findings provide valuable insights into the aroma formation and perception of blue lotus products and offer potential for their application in the fragrance, flavor, and food industries.
Keywords
Nymphaea nouchali var. caerulea
Aromatic plant processed products
HS-SPME-GC-MS Flavoromics Molecular docking
1. Introduction
Water lilies (Nymphaeaceae) are aquatic plants known for their rich nutritional content (Abelti et al., 2023). Introduced to China in the 1970s, they have since undergone hybrid breeding, resulting in varieties with diverse colors and high ornamental value (Zhang et al., 2021). Among them, the blue lotus (Nymphaea nouchali var. caerulea) is a notable species widely distributed across Africa, Australia, and Asia. In these regions, its roots, stems, and flowers have traditionally been used to treat various ailments, including inflammation, fever, and insomnia (Kiranmai et al., 2023). Furthermore, the blue lotus is recognized for its distinctive floral and woody aroma, which has been utilized in aromatherapy, primarily for stress relief and sleep enhancement (Dosoky et al., 2023). Due to its aromatic properties, the blue lotus can be processed into a range of products such as dried flowers, concretes, essential oils, and hydrosols. These products hold promising potential in the cosmetics, perfumery, and food industries.
The unique composition and appropriate concentration of aroma compounds in processed aromatic plant products play a crucial role in defining their characteristic aromas (He et al., 2024). Extensive research has been conducted on processed products (including essential oils, hydrosols, and concretes) derived from popular aromatic plants such as roses, sweet oranges, and oregano (Caputo et al., 2022; Hu et al., 2024; H. Wang, Liu, et al., 2024). Headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME–GC–MS) is widely used for the analysis of volatile compounds in processed aromatic plant products, as it minimizes the negative impact of heating and solvent treatment on their aromatic properties. (H. Xu et al., 2024). The odor activity value (OAV) is commonly used to quantitatively assess the contribution of individual aromatic compounds to the overall aroma, with compounds exhibiting OAV >1 considered key contributors (Tian, Ding, et al., 2023). Wang et al. (Y. Wang, Liu, et al., 2024) utilized HS-SPME-GC-MS combined with OAV analysis to identify key floral-like aroma compounds in green tea, including decanal, linalool oxide, and benzeneacetaldehyde. Furthermore, aroma reconstitution and omission experiments can verify the sensory significance of these key compounds. Xiao et al. (Xiao et al., 2017) successfully simulated the overall aroma of citrus juice through aroma reconstitution experiments, verifying that nonanal, hexanal, linalool, and (R)- (+)-limonene were the key aromatic compounds influencing the overall aroma of juice samples. The combined use of these three methods can be applied to the in-depth study of the volatile compounds and aroma characteristics of blue lotus processed products.
In addition to the aforementioned methods, researchers have employed Partial Least Squares Discriminant Analysis (PLS-DA) to investigate differences in volatile aromatic compounds among products. Correlation analysis has also been applied to link volatile compounds with sensory aroma attributes, aiming to identify key contributors that significantly influence the sensory aroma profile of products (Xiong et al., 2024). Molecular docking techniques have been utilized to explore the potential perception mechanisms of characteristic aromas. Sun et al. (2024) identified key aroma compounds responsible for the honey-like aroma in Zunyi black tea, and further analyzed their interactions with human olfactory receptors through molecular docking. This study provides important theoretical insights for the regulation of characteristic aroma in Zunyi black tea. Based on the experience from the development and aroma characterization of common aromatic plant products, it is evident that the successful development of blue lotus aromatic products requires systematic research on the composition of aroma compounds and sensory aroma characteristics.
The blue lotus is widely distributed worldwide and is valued for its high-quality aroma. However, only a few studies (Dosoky et al., 2023; Tsai et al., 2019) have conducted preliminary analyses of the volatile compounds in blue lotus flowers and concrete, while systematic studies on its processed products remain scarce. Therefore, in this study, three processed products of Chinese blue lotus were prepared using steam distillation and solvent extraction. Their volatile and sensory characteristics were analyzed using HS-SPME-GC-MS and quantitative descriptive analysis (QDA). Key aroma compounds were identified through OAV, aroma recombination and omission tests, and multivariate statistical analysis. In addition, molecular docking was employed to explore the potential mechanisms underlying the perception of characteristic aromas in these products. Our results would help clarify the application potential of blue lotus processed products and provide a valuable reference for the development of novel aromatic plant-based products.
2. Materials and methods
2.1. Materials
Fresh blue lotus flowers were hand-harvested between 8:00 and 10:00 a.m. during July and August 2024 from a cultivation site located at a plantation base in Liwan District, Guangzhou, Guangdong Province, China (He et al., 2024). The flowers were stored under low-temperature conditions (4oC) immediately after harvesting and transported via a high-efficiency cold chain logistics system to the Biotechnology Key Laboratory at Zhengzhou University of Light Industry (Zhengzhou, Henan Province, China). Sample processing is completed within 48 h after picking to prevent sample deterioration and affect the aroma.
2.2. Preparation of blue lotus concrete (NCC), blue lotus essential oil (NCEO), and blue lotus hydrosol (NCH)
Sample preparation was conducted according to a slightly modified procedure previously reported (He et al., 2024). Freshly collected blue lotus flowers were carefully washed, blotted with absorbent paper, and air-dried. As the majority of volatile aroma compounds are concentrated in the petals and stamens (Q. Zhou, Zhao, et al., 2024), these parts (2000 g) were separated, cut into smaller pieces, and oven-dried at 50 °C for 4 h. The dried blue-purple petals and yellow stamens were first ground into powder using an S5-LF960-A mixer (Joyoung Instrument, Hangzhou, China). The powder was then manually sieved through a 20-mesh sieve (0.85 mm pore size) to obtain a uniform and fine blue lotus powder.
NCC was prepared using a solvent extraction method. Given that most aroma compounds in flowers are non-polar, n-hexane (a non-polar, chemically stable, and easily recoverable solvent) was selected for the extraction process (N. Xu et al., 2005). Specifically, 100 g of blue lotus powder was extracted twice with 2 L of n-hexane under magnetic stirring at 200 rpm and a constant temperature of 25 °C for 12 h per cycle. The two resulting extracts were combined and concentrated under vacuum at 40 °C to remove the solvent. NCEO and NCH were prepared by steam distillation. Specifically, 500 g of fresh blue lotus petals and stamens were immersed in 2 L of deionized water and subjected to 4 h of hydrodistillation using Clevenger-type apparatus. The upper oily phase was collected as NCEO, while the lower aqueous gel phase was collected as NCH. NCEO was dried over anhydrous sodium sulphate to remove excess water stains. All final products (NCC, NCEO, and NCH) were vortexed for 20 min to ensure homogeneity, then sealed and stored at 4 °C until further analysis.
The yield of the blue lotus processed products was calculated using the following formula:
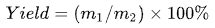
(1)
where m1 represents the weight of the product (g), and m2 represents the weight of the experimental materials (fresh blue lotus petals and stamens, or dried blue lotus powder) (g). The weight of the NCH product (m1) includes both the portion that remained in the apparatus and the portion that refluxed during the distillation process.
2.3. Analysis of volatile compounds using HS-SPME-GC-MS
Volatile compounds in blue lotus and processed products were identified using HS-SPME-GC-MS, based on a slightly modified method described by Yang et al. (S.-B. Yang, Fu, et al., 2024). Specifically, each sample was mixed with the internal standard (phenyl acetate; Macklin, Shanghai, China) and sealed in a 20 mL headspace vial. The vial was immediately capped and equilibrated at 70 °C for 30 min. A manual SPME fiber (50/30 μm DVB/CAR/PDMS; Agilent Technologies, USA) was then inserted into the vial and incubated for an additional 30 min. After extraction, the fiber was desorbed in the injection port of a GC-MS system (7890A-5977B, Agilent Technologies, USA) for analysis.
Helium (99.99 % purity) was used as the carrier gas at a constant flow rate of 1.0 mL/min in splitless mode. The injector temperature was set to 280 °C. The oven temperature program was as follows: an initial temperature of 50 °C (held for 2 min), increased at a rate of 2 °C/min to 250 °C, and held for 10 min.
The mass spectrometry (MS) conditions were as follows: electron impact (EI) ionization at 70 eV, ion source temperature of 230 °C, and a mass scan range of 30–550 amu. The DB-5MS capillary column (30 m × 250 μm × 0.25 μm; Agilent Technologies, USA) was used, with the ion source and transfer line both maintained at 280 °C. A solvent delay of 240 s was applied to reduce column bleed and fiber-related noise, and to protect the ion source (He et al., 2024; Lubes & Goodarzi, 2017). This setting, tailored to samples with medium-to high-boiling volatiles as potential key components. Solvent delay duration should be adapted to sample characteristics and is not universally applicable.
2.4. Qualitative and quantitative analysis of volatile compounds
Qualitative analysis of volatile compounds was performed using mass spectrometry combined with spectral library matching and retention index (RI) comparison. Initial compound identification relied on the NIST 2020 mass spectral library, retaining compounds with a match score greater than 80 %. RIs were calculated using n-alkanes (C6–C30) under identical chromatographic conditions, and verified against literature-based retention index references (RRI).
Quantitative analysis was conducted using a semi-quantitative internal standard method. The concentration of each volatile compound was calculated using the following equation:(2)where C is the concentration of the volatile compound (μg/g), Aw is the peak area of the volatile compound, As is the peak area of the internal standard, Cs is the concentration of the internal standard (μg/g), Vs is the volume of the internal standard (mL), m is the mass of the sample (g), and each sample was analyzed in triplicate.
2.5. Quantitative descriptive analysis (QDA) of sensory attributes
Sensory evaluation was conducted in a quiet, clean, and odor-free room at ambient temperature. A panel of 19 professionally trained assessors (10 females and 9 males) participated in the evaluation. The assessors used a structured scale ranging from 0 (none) to 10 (very strong) to rate the primary sensory aroma attributes of the blue lotus processed products, including floral, citrus, woody, spicy, waxy, fruity, sweet, and herbal notes. The intensity levels were defined as follows: 0 (none), 1 (very weak), 5 (moderate), and 10 (very strong). Each evaluation was performed in triplicate (Jia et al., 2025). The sensory descriptive analysis protocol was reviewed and approved by the Ethics Committee of Zhengzhou University of Light Industry (Zhengzhou, China), and all panel members provided written informed consent prior to participation.
2.6. Identification and verification of key aroma compounds
2.6.1. Identification of key aroma compounds based on OAV
Odor activity value (OAV) is commonly used to assess the contribution of individual aroma compounds to the overall aroma profile of a product. Compounds with OAV greater than 1 are generally recognized as key aroma compounds, as they are present at concentrations exceeding their odor thresholds and thus significantly impact the aroma profile of products.
In this study, OAV were calculated according to the method described by Pei et al. (Pei et al., 2023), using the following equation:(3)Where OAVi is the odor activity value of the compound; Ci is the relative mass concentration of the compound obtained through GC-MS (μg/g); OTi is the odor threshold (μg/g).
For compounds lacking reported odor threshold values (OTi) in publicly available databases or literature, the three-alternative forced-choice (3-AFC) method was used to determine the best estimate threshold (BET), which was then adopted as the OTi. (S. Yang, Fu, et al., 2024). Specifically, each target compound was prepared at an initial concentration of 5 mg/g, and nine concentration gradients were established. Each concentration gradient group consisted of one characterization sample and two blanks (52 % ethanol–water). A trained sensory panel comprising 10 female and 9 male assessors sequentially evaluated each sample set from the lowest to the highest concentration, identifying the characterization sample and recording its coded number. The BET was calculated as the geometric mean of the highest concentration correctly identified by each assessor and the next higher concentration (Jia et al., 2025). All experiments were performed in triplicate.
2.6.2. Aroma reconstitution and omission experiments
In the aroma reconstitution experiment, key aroma compounds (OAV >1) identified in NCC, NCEO, and NCH were used to construct corresponding aroma reconstitution models (NCC-R, NCEO-R, and NCH-R) based on their original mass concentrations in the products (Table S1) (Tian, Lin, et al., 2023). Sensory quantitative descriptive analysis (QDA) was conducted following the procedures outlined in Section 2.5, and sensory attributes of the reconstitution models were compared with those of the original samples to validate aroma similarity.
The aroma omission models were prepared by removing a single compound from the full reconstitution model. Triangle tests were employed to assess sensory differences. Each triangle test consisted of one omission model and two complete reconstitution models, all randomly coded using three-digit numbers. Sensory panelists were asked to identify the sample that was significantly different from the other two based on aroma perception (X. Sun et al., 2021). All omission tests were performed in triplicate at room temperature.
2.7. Molecular docking analysis of key aroma compounds in blue lotus processed products
Human broadly tuned olfactory receptors—OR1A1, OR1D2, OR1G1, and OR2W1—have previously been employed to investigate the perception mechanisms of fruity, floral, sweet, and woody aromas (Sun et al., 2024, Xiao et al., 2024). These receptors are believed to be associated with the detection of floral odorants (Zhu et al., 2024). Based on these findings, these olfactory receptors were selected to explore the potential perception mechanisms underlying the characteristic aroma of blue lotus products. The amino acid sequences of these receptor proteins were retrieved from the UniProt database (https://www.uniprot.org/), and their three-dimensional structures were predicted using AlphaFold (https://alphafold.com/). The molecular structure files of key aroma compounds were obtained in PDB format from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Molecular docking between the aroma compounds and the olfactory receptor proteins was conducted using AutoDock (version 4.2.6, The Scripps Research Institute, USA). Binding energies were recorded after docking, and output files containing detailed positional and interaction data were saved for further analysis. Docking sites and interaction mechanisms were visualized and interpreted using PyMOL (version 3.0, DeLano Scientific LLC, USA).
2.8. Data analysis
Radar charts were generated using Origin 2024 (OriginLab Corporation, USA). Principal Component Analysis (PCA) and Partial Least Squares Discriminant Analysis (PLS-DA) were performed using the online bioinformatics platform (https://www.bioinformatics.com.cn/) and SIMCA 14.1 software. Correlation heatmaps were constructed using OmicStudio (https://www.omicstudio.cn/).
All independent experiments were conducted in triplicate. Quantitative results for volatile compound detection are provided as the mean ± standard deviation (SD).
3. Results and discussion
3.1. Yield and morphological characteristics of blue lotus processed products (NCC, NCEO, and NCH)
Processed products from fresh blue lotus flowers (NCF), including blue lotus concrete (NCC), essential oil (NCEO), and hydrosol (NCH), were obtained through solvent extraction and steam distillation of Chinese-grown blue lotus. The yields and morphological characteristics of the three products are summarized in Table 1. The yield of blue lotus concrete (NCC) was 0.16 %, which is consistent with the 0.18 % reported previously (Dosoky et al., 2023). However, no prior data on the yields of blue lotus essential oil (NCEO) or hydrosol (NCH) have been published. In this study, the yields of NCEO and NCH were 0.03 % and 203.32 %, respectively, which are comparable to those reported for rose-derived products (He et al., 2024). This similarity may be attributed to the use of petals as the primary extraction material in both cases. It is important to note that NCH is a co-product of the essential oil extraction process and primarily consists of condensed steam. Since the steam originates from additional water introduced during distillation rather than from the aromatic plant material itself, a hydrosol yield exceeding 100 % is scientifically reasonable.
Table 1. Yields and form of blue lotus processed products (NCC, NCEO, NCH).
| Sample | aYield (%) | Sample form |
|---|---|---|
| NCC | 0.164 ± 0.010 | dark green, waxy, and viscous texture |
| NCEO | 0.027 ± 0.007 | pale yellow to transparent liquid |
| NCH | 203.321 ± 16.418 | colorless and transparent liquid |
a
The results are the average of three repeated experiments (n = 3).
Understanding the aroma characteristics of processed plant products is essential for product development. To date, research on blue lotus has focused primarily on volatile compound analysis of the flower and concrete (Dosoky et al., 2023; Tsai et al., 2019), while the volatile profiles of essential oil and hydrosol remain largely unexplored. Therefore, in the subsequent stages of this study, emphasis was placed on the characterization of volatile compounds and sensory attributes of these blue lotus processed products to support product development.
3.2. Detection, identification, and PCA of volatile compounds in fresh blue lotus (NCF) and its processed products (NCC, NCEO, and NCH)
Volatile compounds in fresh blue lotus (NCF) and its processed products—blue lotus concrete (NCC), essential oil (NCEO), and hydrosol (NCH)—were identified using HS-SPME-GC-MS. Qualitative and semi-quantitative analyses were performed based on retention indices (RI), a series of n-alkanes (C6–C30), and an internal standard method (Table S1). The composition of volatile compounds was visualized using multiple graphical methods (Fig. 1). A total of 81 volatile compounds were detected across the four samples, including 15 alcohols, 13 ketones, 7 aldehydes, 7 terpenes, 7 esters, 3 phenols, 18 alkanes, and 11 other compounds.

As shown in Fig. 1A, alkanes had the highest relative content in NCF, accounting for 84 %. This is consistent with previous findings showing alkane contents ranging from 48 % to 82 % during different flowering stages (Q. Zhou, Zhao, et al., 2024). In NCC, alcohols were dominant (42 %), with benzyl alcohol being the most abundant compound at 153.77 μg/g. In NCEO, a similar pattern was observed, with alkanes comprising 66 % of the total content and terpenes accounting for 22 %, among which α-farnesene was the most abundant. In contrast, NCH had significantly lower alkane content, likely due to their hydrophobic nature (Q. Zhou et al., 2023), during distillation, alkanes tend to partition into the essential oil (NCEO) rather than the aqueous phase (NCH). While alkanes lack strong or distinctive odors, they contribute to the stability and longevity of aroma in essential oils and aromatic products (Antonova et al., 2021).
As illustrated in Fig. 1B, only 9 volatile compounds were common across all four samples, while most were sample-specific. This suggests that differences in compound composition may arise from various processing methods due to factors such as thermal degradation, volatilization, or solubility variations. Similar findings have been reported for essential oil and hydrosol from Salvia sclarea L. (Clary Sage), where distinct volatile profiles were attributed to solubility differences of the chemical constituents (Acimovic et al., 2022). The same explanation likely applies to the blue lotus products in this study.
Principal Component Analysis (PCA), a multivariate statistical tool for dimensionality reduction and visualization (Feng et al., 2022), was used to assess differences in volatile compounds among the samples (Fig. 1C). In this study, PC1 and PC2 explained 45 % and 32 % of the total variance, respectively, with a combined contribution rate of 77 %. NCEO and NCH samples were in the first and second quadrants, while NCF and NCC samples clustered closely in the third quadrant. This indicates that processing methods significantly influence the volatile compound composition. The similarity between NCC and NCF also suggests that solvent extraction may preserve volatile compounds more effectively. However, 23 % of the variance in the PCA analysis remained unexplained. This may be attributed to pyrolysis during distillation, compound isomerization, or differences in the physical state of the products (waxy, oily, aqueous, or plant-based matrices), which could affect the detection of volatile compounds. Nevertheless, with a confidence threshold exceeding 60 %, the PCA model remains valid for preliminary interpretation and discussion of volatile compound differences (Xie et al., 2022).
Overall, combining HS-SPME-GC-MS with PCA effectively revealed differences in the volatile compound of blue lotus processed products. This approach also helped identify processing methods that better retain the volatile compounds of fresh blue lotus, thereby providing a scientific basis for their optimized application in product development. Certainly, the aromatic characteristics of processed blue lotus products may be influenced by differences in varieties, growing environments and other factors. Future studies should collect samples more extensively and analyze their aroma profiles to ensure the stability of final product quality.
3.3. Identification and analysis of key aroma compounds in blue lotus processed products (NCC, NCEO, and NCH)
In the previous section, the volatile compound composition of blue lotus processed products was preliminarily characterized. In this section, key aroma compounds (OAV >1) were identified based on their odor activity values, as summarized in Table 2. A total of 28 volatile aroma compounds were detected in the three products—20 in NCC, 15 in NCEO, and 16 in NCH. After integrating data from Tables S1 and Table 2, 21 compounds with OAV >1 were considered potential key contributors to the aroma profiles, including 13 in NCC, 12 in NCEO, and 14 in NCH.
Table 2. Fragrance descriptions, odor thresholds, and OAVs of aroma compounds in blue lotus processed products (NCC, NCEO, and NCH).
| No. | Compound | aFragrance | bThreshold (μg/g) | cOAV | ||
|---|---|---|---|---|---|---|
| NCC | NCEO | NCH | ||||
| Terpenes | ||||||
| a1 | trans-α-bergamotene | citrus, floral, warm | 2.500 | 82.497 | ||
| a2 | β-caryophyllene | candy, citrus, herb, | 0.064 | 3.175 | ||
| a3 | α-curcumene | herb, honey, lemon | 5.000 | <1 | <1 | |
| a4 | α-farnesene | boiled vegetable, citrus, floral, herb | 0.020 | 32206.700 | ||
| a5 | β-sesquiphellandrene | herb, wood | nd | – | ||
| a6 | trans-α-bisabolene | balsamic, citrus, herb | 10.000 | <1 | <1 | <1 |
| Aldehydes | ||||||
| b1 | benzaldehyde | almond, berry | 0.350 | 18.790 | 28.390 | 128.905 |
| b2 | octanal | citrus, fat, fruit, honey | 0.001 | 2198.291 | ||
| b3 | nonanal | fat, floral, | 0.001 | 3054.862 | ||
| b4 | 4-methoxybenzaldehyde | anise, caramel, sweet | 0.030 | 23.397 | 130.916 | |
| b5 | 2-phenyl-2-butenal | honey, nut, popcorn | 3.000 | <1 | ||
| Alcohols | ||||||
| c1 | benzyl alcohol | boiled cherries | 20.000 | 7.688 | 3.517 | |
| c2 | 4-terpineol | earth, herb, woody | 1.200 | <1 | 2.178 | 3.800 |
| c3 | myrtenol | woody | 0.007 | 236.242 | ||
| c4 | lev verbenone | woody | 15.000 | <1 | <1 | <1 |
| c5 | 4-methoxybenzyl alcohol | anise, herb, sweet | 38.000 | <1 | ||
| c6 | d-nerolidol | floral | 0.100 | 53.566 | ||
| c7 | cedrol | sweet, woody | 18.170 | <1 | ||
| Ketones | ||||||
| d1 | 6-methyl-5-hepten-2-one | fruit, green, | 0.050 | 32.968 | 41.087 | |
| d2 | α-ionone | floral, woody | 0.001 | 14989.280 | 20729.290 | 14900.110 |
| d3 | dihydro-β-ionone | floral, fruit, woody | 0.004 | 1946.617 | 5833.255 | |
| d4 | geranyl acetone | hay, fruity | 0.060 | 12.460 | 16.083 | |
| d5 | β-ionone | woody, dry, floral, | 0.000 | 5901115 | 30320346 | 6300913 |
| Phenols | ||||||
| e1 | 2-methoxy-4-vinylphenol | burnt, clove, cooked | 0.003 | 3867.919 | ||
| Esters | ||||||
| f1 | acetic acid benzyl ester | boiled vegetable, fruit, honey | 0.364 | 2.757 | 19.219 | 48.647 |
| f2 | ethyl 4-methoxybenzoate | anise, fennel | 0.152 | 10.117 | ||
| f3 | methyl jasmonate | floral | 0.070 | 42.091 | 19.313 | |
| f4 | benzyl benzoate | balsamic, herb | 0.340 | 30.971 | 59.846 | 19.195 |
a
Fragrance descriptors sourced from www.vcf-online.nl/VcfHome.cfm.b
Odor thresholds from www.vcf-online.nl/VcfHome.cfm andthe article (Gemert, 2003). If unavailable, thresholds were estimated via the three-alternative forced-choice (3-AFC) (Z. Wang et al., 2024). “nd” indicates that the odor threshold was not found or could not be determined.c
A blank space indicates that the compound is absent in the sample, while a dash (“—”) indicates that the odor threshold was not calculated.
In the NCC sample, β-ionone, dihydro-β-ionone, and α-ionone exhibited especially high odor activity values (OAV), owing to their relatively high concentrations (Table S1) and extremely low odor thresholds (Table 2). These compounds, characterized by their floral and woody odor profiles (Table 2), are likely to play a pivotal role in shaping the distinctive aroma of the NCC product. In addition, several other compounds with OAV >1 were detected in NCC, including benzaldehyde, 4-methoxybenzaldehyde, ethyl 4-methoxybenzoate, geranyl acetone, d-nerolidol, methyl jasmonate, and benzyl benzoate. The presence of these compounds is likely to enhance the floral, fruity, sweet, and herbal nuances of the NCC aroma profile.
In the NCEO sample, compounds with particularly high OAVs included β-ionone, dihydro-β-ionone, and α-farnesene. This differs from rose essential oil and related products, where floral and woody notes are primarily attributed to rose oxide, β-citronellol, linalool, α-terpineol, and D-limonene (He et al., 2024). In contrast, the floral and woody characteristics of NCEO are more likely derived from β-ionone and dihydro-β-ionone. In addition, NCEO also contains octanal, which contributes a citrus-like aroma. Notably, octanal was exclusively detected in NCEO, but was absent in NCC or NCH. This selective presence is likely due to its stronger lipophilicity resulting from longer hydrocarbon chains (Frank et al., 2017), which favors its enrichment in the essential oil phase during steam distillation. NCEO also contains β-sesquiphellandrene, a compound with strong herbal and woody notes, as well as reported bioactivity (Tyagi et al., 2015). Although its odor threshold remains undetermined, its high concentration suggests a potentially significant contribution to the NCEO aroma profile. This compound has also been previously detected in blue lotus (Dosoky et al., 2023).
In the NCH sample, β-ionone, dihydro-β-ionone, and α-ionone again exhibited the highest OAVs, suggesting that floral notes may dominate the aroma of this hydrosol product. Additionally, 4-methoxybenzaldehyde and acetic acid benzyl ester may contribute sweet, fruity, and herbal-like nuances. A particularly notable compound in NCH is 2-methoxy-4-vinylphenol (OAV = 4054.5218), which is associated with clove-like, cooked, and warm aromas, and may be responsible for the unique aroma profile of NCH. Interestingly, the major terpene compounds identified in NCEO were not detected in NCH. This may be attributed to the fact that NCH is the aqueous phase during steam distillation, whereas terpenes are mostly lipophilic (Masyita et al., 2022) and therefore tend to partition into the essential oil phase (NCEO) rather than remain in the water-rich hydrosol fraction.
3.4. Results and analysis of aroma reconstitution and omission experiments
To validate the accuracy of key aroma compounds identified through the OAV method, reconstitution models were established for each processed product (NCC, NCEO, and NCH) using the respective aroma compounds with OAV >1 (Table S2) (Tian, Lin, et al., 2023). Sensory evaluations were conducted following the quantitative descriptive analysis method described in Section 2.5, and differences between the reconstitution models (NCC-R, NCEO-R, NCH-R) and their corresponding original samples were compared, as shown in Fig. 2.

As shown in Fig. 2A, the aroma profiles of NCC and its reconstituted model (NCC-R) were nearly identical, indicating that the selected key compounds could effectively simulate the original aroma. In contrast, as shown in Fig. 2B, the reconstituted model NCEO-R exhibited a noticeably weaker woody note compared to NCEO. This difference may be attributed to the absence of β-sesquiphellandrene—a compound with a distinct woody aroma—in the reconstitution model (Table S2). Due to the limited availability of this compound, its contribution to the overall aroma could not be directly confirmed in the reconstitution experiments. However, the potential key roles of this compound in aroma formation were indirectly confirmed through subsequent omission experiments (Table S3). Future studies could employ gas chromatography–olfactometry (GC-O) and related techniques to further quantify the actual impact of such compounds on aroma formation. Finally, as shown in Fig. 2C, the aroma profiles of NCH and NCH-R were also highly consistent, indicating successful aroma reconstruction for the hydrosol product.
The aroma omission experiment, conducted based on the aroma reconstitution model, further validates the accuracy of the OAV method in identifying key aroma compounds (Ma et al., 2017). The results (Table S3) demonstrate that omitting key aroma compounds identified via the OAV method had varying degrees of impact on the perceived aroma profiles. Notably, omission of β-ionone, dihydro-β-ionone, and α-ionone in all three products led to highly significant decreases in floral and woody notes (P ≤ 0.001), suggesting that these ionone-type compounds are major contributors to the sensory characteristics of the processed products.
In some cases, however, a portion of the assessors failed to distinguish the reconstitution model from the omission model upon the removal of specific compounds, such as benzyl benzoate in NCC and NCEO, and geranyl acetone in NCH. This could be attributed to several factors, including: individual differences in olfactory sensitivity (Keller et al., 2007), odor masking effects caused by stronger compounds (Z. Wang et al., 2024), and relatively low aroma activity values of these compounds in the sample matrices. While this phenomenon had no significant impact on the overall conclusions, its underlying causes were not extensively investigated in this study.
3.5. Correlation analysis of volatile compounds and aroma attributes in blue lotus processed products (NCC, NCEO, and NCH)
PLS-DA analysis was conducted to reveal differences in volatile compound profiles among the three blue lotus processed products, with the aim of accurately identifying the compounds that distinguish the samples. As shown in Fig. 3A and B, the PLS-DA model constructed based on volatile compound contents demonstrated excellent fit (R2 = 0.99) and strong predictive performance (Q2 = 0.98). The results of the 200-time permutation test (R2 = 0.107, Q2 = −0.323) confirmed that the model was not overfitted and could discriminate among the three product (P. Sun et al., 2024). Based on the PLS-DA results, 16 volatile compounds with VIP values greater than 1 (Fig. S1) were identified. These compounds contributed significantly to product differentiation and were defined as differential volatile compounds (VIP >1).

Quantitative Descriptive Analysis (QDA) was performed to evaluate eight aroma attributes—floral, citrus, woody, spicy, waxy, fruity, sweet, and herbal. The QDA results (Fig. 3C) showed distinct aroma attributes among the three samples. NCC exhibited strong sweet and waxy attributes, which align with previous findings on the waxy characteristics of processed aromatic plant products (Kang et al., 2022; Trovato et al., 2024). NCEO was dominated by herbal, floral, citrus, and woody attributes, likely due to the presence of high-OAV compounds such as trans-α-bergamotene (citrus, floral) and β-ionone (floral, woody). Woody, floral, and citrus notes are currently among the most popular fragrance profiles in the perfume market. The distinctive aromatic characteristics of NCEO endow it with the potential to impart high-quality, natural signature scents to perfume products. As a component of fragrance formulations, NCEO offers additional options for the creation of premium perfumes. In contrast, NCH was characterized by pronounced spicy and fruity attributes, possibly resulting from high-OAV compounds like 2-methoxy-4-vinylphenol and acetic acid benzyl ester, both associated with cooked or burnt aromas. Hydrosols are perceived as more natural and safer compared to synthetic additives, which makes them more likely to gain consumer favor (D’Amato et al., 2018). The aromatic profile of NCH also endows it with the potential for flavoring food products. Moreover, as a by-product of essential oil extraction, hydrosols contain trace amounts of essential oils and are more readily available, potentially with lower toxicity (Al-Juhaimi et al., 2025). In the future, it would be worthwhile to investigate the bioactivity of NCH and explore its potential as a natural food preservative.
Among the differential volatile compounds (VIP >1), 9 aroma compounds also exhibited OAV >1 (Table 2). These compounds were considered as the main contributors to the differences of aroma attributes among the three products. A Spearman correlation analysis was performed between the concentrations of these compounds and the QDA results (Fig. 3D). The results showed that all 9 compounds had significant correlations with one or more attributes (P < 0.05) and were defined as key differential aroma compounds (VIP >1, OAV >1, P < 0.05) (Ye et al., 2025).
Among them, the NCC sample contained the highest concentration of benzyl alcohol (Table S1), which showed a strong positive correlation with sweet attributes (P < 0.001) (Yin et al., 2022), consistent with its highest sweet attribute score in QDA (Fig. 3C). Additionally, β-ionone, β-sesquiphellandrene, trans-α-bergamotene, and α-farnesene showed significant positive correlations with floral, woody, herbal, and citrus attributes (P < 0.05 or P < 0.01), while exhibiting significant negative correlations with fruity and sweet attributes (P < 0.01). These findings are consistent with previous reports that emphasized the contributions of β-ionone (Yu et al., 2021) and α-farnesene (Lin et al., 2022; T. Zhou et al., 2024) to woody, floral, and citrus attributes. These compounds were detected at high concentrations in the NCEO sample (Table S1), likely explaining its high QDA scores for herbal, woody, floral, and citrus attributes and low scores for sweet and fruity (Fig. 3C). 2-methoxy-4-vinylphenol (Yao et al., 2021), acetic acid benzyl ester, and benzaldehyde showed significant positive correlations with the spicy attribute (P < 0.01 or P < 0.001) and significant negative correlations with the waxy attribute (P < 0.05, P < 0.01, or P < 0.001). These compounds were most abundant in the NCH sample (Table S1), which likely explain its prominent spicy attributes and minimal waxy attributes. Meanwhile, NCH had the highest concentration of α-ionone, which previously demonstrated to have a positive correlation with the fruity attribute (P < 0.01) (Liu et al., 2021), possibly contributing to the high fruity score of this sample (Fig. 3C). In rose hydrosol, apart from phenylethyl alcohol and geraniol, which impart sweetness and rose floral notes, the concentrations of other volatile compounds are relatively low (He et al., 2024). This limitation may result in a less complex and rich aroma profile of the product. In contrast, blue lotus hydrosol contains a greater variety and higher concentrations of aromatic compounds, leading to a more complex and richer aroma profile, making the product application scenarios more extensive.
3.6. Molecular mechanisms of characteristic aroma perception in three processed products
The woody and floral characteristics of aromatic products contribute to a superior aromatic experience for consumers (Errajaa et al., 2020). As shown in the Quantitative Descriptive Analysis (QDA) results (Fig. 3C), all blue lotus three processed products exhibited favorable performance in terms of woody and floral aroma attributes, indicating their potential application in fields such as food and cosmetics. In previous sections, 4 aroma compounds—β-ionone, β-sesquiphellandrene, trans-α-bergamotene, and α-farnesene—were identified as significantly positively correlated with woody and floral attributes. Among them, β-ionone was present in all three products (NCC, NCEO, and NCH), while the other three compounds were detected only in NCEO. To investigate the molecular mechanisms involved in the perception of woody and floral characteristics, molecular docking was conducted between these 4 aroma compounds and 4 broad-spectrum human olfactory receptors (OR1A1, OR1D2, OR1G1, and OR2W1)(Sun et al., 2024; Xiao, Shen, et al., 2024).
The binding energy heatmap of the 4 aroma compounds with olfactory receptors (Fig. S2) shows that the binding energies between these aroma compounds and the 4 broad-spectrum olfactory receptors are all below 0 kcal/mol, indicating that these compounds to more easily activate the olfactory receptors and express their characteristic odors (Xiao, Li, et al., 2024). Consequently, these compounds play a crucial role in the woody and floral aroma attributes of the blue lotus processed products. Among the 4 olfactory receptors, OR1D2 exhibits the lowest average binding energy (Fig. S2). Therefore, the interactions between the aroma compounds and olfactory receptors in the three processed products were analyzed using OR1D2 as a representative model. Additional molecular docking results with other olfactory receptors (OR1A1, OR1G1, and OR2W1) are provided in Fig. S3–S5 to further elucidate the molecular mechanisms underlying the perception of woody and floral aroma attributes.
Fig. 4 shows the binding interactions of 4 aroma compounds with the human olfactory receptor OR1D2. Fig. 4A illustrates the binding of β-ionone with OR1D2. When β-ionone binds to the olfactory receptor, in addition to forming hydrophobic interactions, the oxygen atom in its ketone group also forms a hydrogen bond with the amino acid residue TYR 182 on the receptor. Hydrogen bonding has been considered a key force in stabilizing the characteristic aroma of products in previous studies (Sun et al., 2024). Additionally, π-π stacking, which belong to hydrophobic interactions can further enhance the hydrogen bonding effect (Mignon et al., 2005; Zhu et al., 2025), stabilizing the conformation of the odorant-receptor binding. This provides a reasonable explanation for the lowest binding energy of β-ionone.

From Fig. 4B to D, it is evident that β-sesquiphellandrene, trans-α-bergamotene, and α-farnesene primarily form hydrophobic interactions when binding with OR1D2. Previous studies have shown that when odorants and olfactory receptors form a more complete hydrophobic region with hydrophobic amino acid residues, the stability of the binding conformation is ensured, which facilitates the formation of the characteristic aroma of the odorant (Sun et al., 2024; Zeng et al., 2023). This also indicates that hydrophobic interactions play a crucial role in the perception of woody and floral aroma in the blue lotus processed products. Notably, β-ionone is present in all three processed products, while β-sesquiphellandrene, trans-α-bergamotene, and α-farnesene are only found in the NCEO product. This suggests that when perceiving the aroma of the products, more aroma compounds significantly positively correlated with woody and floral aroma are detected in NCEO, which may explain why the NCEO sample scored higher for woody and floral attributes compared to the NCC and NCH samples (Fig. 3).
4. Conclusion
In this study, Nymphaea nouchali var. caerulea (blue lotus) cultivated in Guangdong, China, was processed into concrete (NCC), essential oil (NCEO), and hydrosol (NCH) via solvent extraction and steam distillation, with NCH exhibiting the highest yield (203.321 ± 16.418 %). Principal component analysis (PCA) revealed significant differences in volatile compositions among the products, with NCC showing the greatest similarity to the fresh flower. Based on odor activity values (OAV), aroma recombination/omission experiments, and partial least squares-discriminant analysis (PLS-DA), nine key aroma compounds (VIP >1, OAV >1) were identified. Notably, four of which- β-ionone, β-sesquiphellandrene, trans-α-bergamotene, and α-farnesene – were strongly correlated with floral and woody sensory attributes. Molecular docking further demonstrated that these compounds stably bind to human olfactory receptors, primarily through hydrophobic interaction. In addition, β-ionone also formed hydrogen bonds to further enhance the stability of the binding. These findings enhance understanding of the sensory profiles and molecular mechanisms of aroma perception in blue lotus products, supports the utilization of aromatic plant resources and the development of related products and their potential applications in the fragrance, flavor, and food industries.
Credit authorship contribution statement
Pan Wu: Writing – review & editing, Writing – original draft, Investigation, Data curation. Dongyue Zong: Project administration, Methodology. Lei Yang: Supervision, Project administration. Xuewei Jia: Writing – review & editing, Project administration. Lili Qu: Methodology, Data curation. Yihong Wu: Writing – review & editing, Data curation. Chunping Xu: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the 2024 Science and Technology Planning Project of Henan Province [242102311257] the Major Science and Technology Project of China National Tobacco Corporation [110202201005 (JY-05)].