Abstract
This study aims to enhance the stability and bioavailability of nuciferine (NF) and epigallocatechin-3-gallate (EGCG) by loading NF into liposomes and then incorporating the liposomes and EGCG into porous microgels (NFEG-microgel) prepared with chitosan and proanthocyanidin. Analysis of particle size (0.5–3.0 μm), electron microscopy, rheology, stability, and simulated gastrointestinal release confirmed that the prepared microgels had high encapsulation rate and good stability and release characteristics. Intervention experiments were performed by orally administering NFEG-microgel to high-fat diet rats to evaluate its efficacy and regulatory mechanism for blood lipid metabolism. NFEG-microgel intervention significantly reduced the body weight and serum lipid level, and the mechanism was related to the expression regulation of key genes involved in lipid metabolism and miRNAs (miR-126a-5p and miR-30b-5p) in serum extracellular vesicles. In addition, NFEG-microgel improved the diversity of gut microbiota by enriching short-chain fatty acids (SCFA)-producing bacteria and reducing harmful bacteria, suggesting that it can ameliorate lipid metabolism by regulating the intestinal flora community in rats.
Keywords: nuciferine, epigallocatechin-3-gallate, microgel, miRNAs, lipid metabolism genes, gut microbiota
Graphical Abstract
NFEG-microgel was prepared and intervention experiments were performed by orally administering NFEG-microgel to high-fat diet rats to evaluate its efficacy and regulatory mechanism for blood lipid metabolism. The amelioration of lipid metabolism was relative to regulating the expression of key genes which interacted with miR-126a-5p and miR-30b-5p in serum extracellular vesicles and the intestinal flora community in rats. 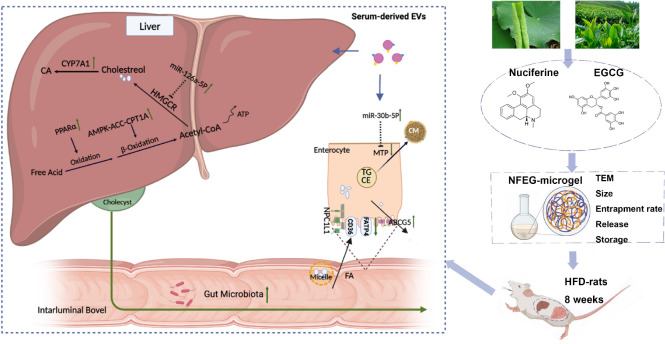
Introduction
Nuciferine (NF, PubChem CID: 10146) is an aromatic ring-containing alkaloid extracted from Nelumbo nucifera leaves and has a wide variety of pharmacological activities, such as lipid lowering (1), anti-inflammatory, antioxidant, anti-liver injury, and anticancer (2). NF also inhibits the accumulation of lipid in 3T3-L1 preadipocytes by regulating the expression of lipid gene (3) and improves the lipid profile in mouse diabetic model by activating peroxisome proliferator-activated receptors-α (PPAR-α)/peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α) (4). In addition, NF treatment changes the composition of the intestinal microbiota of rats fed with high-fat diet (HFD) (5).
Epigallocatechin-3-gallate (EGCG), the major polyphenolic catechin of green tea, is the most abundant antioxidant catechin accounting for about 50∼80% of total catechins content. Although the biological effects of EGCG have not been completely discovered, it can enhance lipid metabolism, has anti-inflammatory and antioxidant activities, and can reduce tumor incidence. Increasing evidence indicates that EGCG has a wide range of biological activities, including activating AMP-activated protein kinase (AMPK), promoting lipid metabolism, and improving insulin resistance, which has a multipronged preventive and therapeutic effect on non-alcoholic fatty liver disease (NAFLD) (6). In addition, EGCG can improve different types of liver injury (7).
Nuciferine has extremely low water solubility and dispersion, and its oral bioavailability is only 3.9% (8). Its stability, bioavailability, and absorptivity can further decline due to gastrointestinal conditions, which is similar to the trend of the stability and bioavailability of EGCG. In humans, the maximum plasma concentration of EGCG is only 0.15 μM after consuming two cups of green tea (4). The double-layer encapsulation of liposome and microgel is adopted to improve the stability and bioavailability of NF and EGCG. In this process, NF and EGCG are encapsulated simultaneously to play a synergistic role at different points in the metabolic cycle. Microgels (d. 1∼350 μm) are swellable polymer networks with hydrophilic functionalities that allow them to entrap large amounts of water without collapse and are usually prepared by polymer (polysaccharides and/or proteins) cross-linking through ionic, physical, or covalent interactions (9). Microgels have been widely used in the encapsulation of various unstable substances, such as astaxanthin (10) and polyphenols (11), to improve their stability and bioavailability and control their release. Myrica rubra leaf proanthocyanidin extract (MLPE) and chitosan are abundant natural materials widely used in the food industry because of their safety and good functional properties. Chitosan coating can increase the stability of liposomes in various biological fluids including simulated gastric fluid (SGF) and simulated small intestinal fluid (SIF), the adhesion of mucosa, and the solubility of drugs (12).
Lipid metabolism is a process of lipid synthesis and degradation in cells, including lipid decomposition, storage for energy and synthesis of structural and functional lipids. Its main function is to transport lipids to the peripheral tissues for use or to transport lipids back to the liver for recycling and removal. And their disorders will lead to the occurrence of many diseases that seriously endangers people’s life and health. According to the functional division, lipid metabolism is roughly composed of three parts: exogenous lipid absorption, endogenous lipid synthesis, and adverse cholesterol transport (13). Lipid metabolism disorders lead to the increase of total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C), and the decrease of high-density lipoprotein cholesterol (HDL-C) which can induce a variety of diseases such as non-alcoholic fatty liver disease and varying evolutions. The liver is a vital organ of the regulation of lipid steady state. Excessive accumulate of lipid in the liver and overloaded liver cause subsequent series of inflammation, insulin resistance, oxidative stress, mitochondrial dysfunction, and intestinal flora disturbance, in the occurrence and development of NAFLD, resulting in multiple attacks on the liver (14). And the published literatures show that changes in the expression or activity of miR-34a, miR-33, miR-122, and miR-21 are the key mechanisms responsible for the development of NAFLD and its progression toward a serious status (15). Animal studies elucidated that the intestinal microbiota, which is consistently rich in proteobacteria, may have a causal role in NAFLD (16).
In our study, NFEG-microgel was prepared, characterized, and used to prevent and inhibit dyslipidemia or NAFLD. Its regulating mechanism for lipid metabolism disorder was also investigated and clarified.
Materials and methods
Materials and instruments
Nuciferine was bought from MedChemExpress Co., Ltd. (Shanghai, China, purity > 98%). Standard EGCG was obtained from Beijing Solarbio Technology Co., Ltd. (Beijing, China, purity > 98%). Chitosan of medium molecular weight was purchased from Sigma–Aldrich (St. Louis, MO, USA, purity > 75%). Lipo3000 transfection reagent was acquired from Thermo Fisher Co., Ltd. TRIzol® Plus RNA Purification Kit and Transcriptor First Strand cDNA Synthesis Kit were bought from Thermo Fisher (Waltham, MA, USA). Dual-luciferase reporter assay system was purchased from Promega Co., Ltd. (Madison, WI, USA).
The following instruments were used in this study: Zetasizer Nano ZS90 (Malvern Instruments Co., Ltd., Malvern, UK), MIKRO 22R centrifuge (Kirchlengern, NRW, Germany), OLYMPUS BX60 fluorescent microscope camera (OLYMPUS Corporation, Tokyo, Japan), transmission electron microscope (TEM) (Hitachi, Tokyo, Japan), and CFX384 multiplex real-time fluorescence quantitative PCR instrument (Bio-Rad, Hercules, CA, USA).
Manufacturing of nuciferine-loaded liposomes
Nuciferine-loaded liposomes (NF-liposomes) were manufactured by solvent evaporation and ultrasonic homogenization following a previous method (17) with some modifications. In brief, 1 g of soybean phospholipids (Shanghai Taiwei Pharmaceutical Co., Ltd., Shanghai, China) and 0.2 g of cholesterol (Shanghai Titan Chemical Co., Ltd., Shanghai, China) were dissolved in trichloromethane and stirred with magnetic force. After complete distribution, 0.2 g of NF dissolved in methanol was added and the mixture was stirred evenly. The organic solvent was evaporated by rotation (30 rpm) at 30°C to form a light-yellow film, which was then dispersed with 0.05 M phosphate buffered saline (PBS). Ultrasonic crushing was performed in an ice bath for 6 min to obtain a translucent liposome solution. Finally, particle size, zeta potential, and polydispersity index (PDI) were analyzed by Zetasizer Nano ZS90. Encapsulation rate was determined by high-performance liquid chromatography (HPLC).
Preparation of chitosan-procyanidin microgels loaded with nuciferine and epigallocatechin-3-gallate
Chitosan–procyanidin microgels were prepared following the methods of Zhang et al. (18) and Zou et al. (19) with some modifications. Chitosan was dissolved in 1% acetic acid solution to a concentration of 6 mg/ml. The liposome solution loaded with NF was mixed with chitosan solution, and magnetic stirring was performed for 1 h. MLPE with purity of 70% and average polymerization degree of 6.7 was extracted in our lab. The two concentrations of MLPE (2 and 6 mg/ml) were added to the above solution (EGCG was dissolved in advance so that the final concentration was 2 mg/ml), and magnetic stirring was performed for at least 3 h to obtain NF-EGCG double-encapsulated microgel (NFEG-microgel). After overnight refrigeration, the encapsulation rate and stability of the two concentrations of MLPE microgels were compared. The results showed that the encapsulation rate was high and stable upon the addition of 2 mg/ml MLPE in the microgel. Thus, subsequent microgels were prepared with this concentration.
Characterization
Encapsulation efficiency
After the preparation of microgels, encapsulation efficiency was determined by ultrafiltration centrifugation. In brief, 0.5 ml of NFEG-microgel solution was centrifuged in an ultrafiltration centrifuge tube at 4,000 g for 30 min. The liquid supernatant of the outer tube, which contained unencapsulated NF and EGCG, was obtained. The content of NF and EGCG was determined by HPLC, and the encapsulation efficiency of NFEG-microgel was calculated according to the following formula:
| Encapsulationefficiency=𝑊1−𝑊2𝑊1×100%, |
where W1 is the total NF or EGCG weight of NFEG-microgel, and W2 is the weight of NF or EGCG in the supernatant.
Morphological observation
The sample was first dispersed and dropped onto a carbon coppered grid, and the morphological characteristics of the microgel prepared with chitosan and MLPE were photographed by TEM.
Average particle size, polydispersity index, and zeta potential
Particle size was measured by LS13 320 Laser particle size analyzer (Beckman coulter, Brea, CA, USA), and zeta potential, and PDI were analyzed by Zetasizer Nano ZS90 after the sample was dispersed.
Rheological property analysis
The rheological properties of samples were determined using Discovery Hybrid Rheometer (TA Instruments, Wilmington, DE, USA) (20). The measured shear rate (τ) increased from 0.01 to 500 s–1 and was used to evaluate the viscosity and shear stress of the microgel.
Simulated gastrointestinal digestion of NFEG–microgel in vitro
In brief, 5 ml of microgel was first digested in 5 ml of SGF with pH of 2.0 for 2 h, followed by the addition of 10 ml of SIF and incubation for 6 h. The pH was adjusted to 7.0 with 0.1 M NaHCO3 solution following a previous method with some modifications (11). Incubation was carried out in a shaker under 120 rpm/min and 37°C. The samples were extracted at 0.5, 1, 2, 4, 6, and 8 h, and the same volume of release media was added. SGF composed of 0.9 g of NaCl and 0.14 g of pepsin (1:3,000, Beijing Solarbio Technology Co., Ltd., Beijing, China) was dissolved in 100 ml of 0.1 mol/L HCl aqueous solution at a final pH of 2.0. The simulated SIF was composed of 0.225 g of trypsin (1:250, Hefei Bomei Biotechnology Co., Ltd., Hefei, China) and 1.125 g of pig bile salt (China National Institute for Food and Drug Control, Beijing, China).
Storage stability
Nuciferine solution dissolved in 0.5% sodium carboxymethyl cellulose, EGCG solution, and NFEG-microgel were stored at 4°C for 12 weeks at their corresponding concentrations. Their storage stability was evaluated by their apparent appearance and encapsulation rate (21).
Animal sample collection and processing
Thirty healthy male Wistar rats (180 ± 20 g) with special pathogen free were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China) with animal license number SCXK (Shanghai, China) 2017-0005. The rats were raised in standard conditions (temperature 25 ± 1°C, relative humidity 40–60%, and 12 h light/dark cycles) and had free access to food and water. Their dietary consumption was recoded daily. All animal procedures were permitted by the Laboratory Animal Ethics Committee (2022-005) of China Jiliang University (Hangzhou, China). After a week of acclimatization, the rats were randomly divided into the following six groups with five rats each: blank control (NC), HFD, NF-loaded microgel treatment with high-fat diet (HFD + NF), EGCG-loaded microgel treatment with HFD (HFD + EGCG), NF and EGCG co-loaded microgel treatment with HFD (HFD + NFEG), and empty microgel treatment with HFD (HFD + MG). The NC group received commercial normal diet, and the remaining groups received HFD purchased from Fanbo Animal Feed Biotechnology Co., Ltd. (Shanghai, China) and consisted of 10% lard, 10% yolk powder, 6% casein, 3% maltose, 2% cholesterol, 1.2% premix, and 0.1% cholate. The rats in HFD + NF group intragastrically received NF at 20 mg/kg/day, those in HFD + EGCG group intragastrically received EGCG at 20 mg/kg/day (22), and those in HFD + NFEG group intragastrically received the same NF (20 mg/kg/day) and EGCG (20 mg/kg/day) for 8 weeks (23), The volume of the treatments was 10 ml/kg of rat body weight. The rats in NC and HFD + MG groups intragastrically received the same volume of water and empty microgel, respectively, for 8 weeks. Food intake was recorded daily, and body weight was monitored weekly. Blood samples were collected from the tail every 2 weeks and then centrifugated at 1,500 × g for 15 min at 4°C to separate the serum. The biochemical indexes of the serum were detected using the corresponding kits. Triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL-C), and low-density lipoprotein (LDL-C) kits were purchased from Thermo Fisher Co., Ltd. (Waltham, MA, USA). At the end of the experiment, the rats were sacrificed. Blood, liver, small intestine, and cecum contents were obtained and stored at –80°C. The liver was sectioned, preserved in formalin fixative, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E).
Real-time quantitative PCR analysis
Total RNA of liver and small intestine tissues was extracted using TRIzol® Plus RNA Purification Kit following the manufacturer’s protocol. Single-stranded cDNA was synthesized with the Transcriptor First Strand cDNA Synthesis Kit. Real-time quantitative PCR (RT-PCR) was conducted to determine the expression of PPARα, cholesterol 7 alpha-hydroxylase A1 (CYP7A1), 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), adenosine monophosphate activated protein kinase (AMPK), ACC in the liver, carnitine palmitoyl transferase 1A (CPT1A), and ATP-binding cassette G5 (ABCG5), fatty acid translocase CD36 (CD36), Niemann-Pick C1-like 1 (NPC1L1), fatty acid transport protein 4 (FATP4), and microsomal triacylglycerol transfer protein (MTP) in the small intestine mRNA. Quantitative PCR primers were designed using Primer Premier 6.0 and Beacon Designer 7.8 and synthesized by Shenggong Bioengineering Co., Ltd. (Shanghai, China). RT-PCR was performed with PowerUp™ SYBRTM Green Master Mix (Applied Biosystems, Foster City, CA, USA) using CFX384 Real-Time Fluorescence Quantitative PCR System (Bio-Rad, Hercules, CA, USA). The primer sequences are shown in Table 1. The reaction conditions were as follows: 95°C for 1 min and 40 cycles of 95°C for 15 s and 63°C for 25 s to collect fluorescence. The expression of genes was normalized in reference to the housekeeping gene GAPDH, and the relative gene expression in the six groups was statistically analyzed using the 2–ΔΔCt method.
TABLE 1.
Real-time PCR primers in rat liver and small intestine.
| Gene | GenBank accession | Reverse transcription primer sequences (5′–3′) | Size (bp) |
| Rat GAPDH | NM_017008.4 | GAAGGTCGGTGTGAACGGATTTG | 127 |
| CATGTAGACCATGTAGTTGAGGTCA | |||
| Rat PPARα | NM_013196.2 | GGAGGCAGAGGTCCGATT | 131 |
| TCAGCAAGGTAACCTGGTCATTCAA | |||
| Rat CPT1A | NM_031559.2 | GCACATTAGACCGTGAGGAACT | 138 |
| CCTTGATATGTTGGATGGTGTCTGT | |||
| Rat ACC | NM_022193.1 | GAGGTTGGCTATCCAGTGATGA | 102 |
| CTGTCTGAAGAGGTTAGGGAAGT | |||
| Rat CYP7A1 | NM_012942.2 | CAAGACGCACCTCGCTATTCTCT | 113 |
| CTTCAGAGGCTGCTTTCATTGCT | |||
| Rat HMGCR | NM_013134.2 | CCTGCGTGTCCCTGGTCCTA | 125 |
| CCTTTGGGTTACTGGGTTTGGT | |||
| Rat AMPK | XM_008763901.1 | GATTTGCCCAGTTACCTCTTTCC | 156 |
| CACTGCGAGCTGGTCTTGA | |||
| Rat CD36 | AF072411.1 | CGGTTGGAGACCTACTCATTGA | 147 |
| CCACTTCCTCTGGGTTTTGC | |||
| Rat FATP4 | NM_001100706.1 | CCTCTACCACTCAGCAGGAAA | 156 |
| CGGCAAAGCTCACCAATGTAC | |||
| Rat ABCG5 | NM_053754.2 | CTTCTGTGCCAAATAACCCAATG | 135 |
| GGATGACAAGAGTCGGGATGAA | |||
| Rat NPC1L1 | NM_001002025.1 | GCTGCTGTTTCTGACCCTGTTT | 141 |
| CCCACTTCAAGGTATCGGTTCAG | |||
| Rat MTP | NM_001033694.1 | GTTCTCCCAGTACCCGTTCTTGGT | 100 |
| CCTCCCTGTGGATAGCCTTTCAT |