Abstract
A quantitative analytical method for five aporphine alkaloids, nuciferine (1), nornuciferine (2), N-methylasimilobine (3), asimilobine (4), and pronuciferine (5), and five benzylisoquinoline alkaloids, armepavine (6), norarmepavine (7), N-methylcoclaurine (8), coclaurine (9), and norjuziphine (10), identified as the constituents responsible for the melanogenesis inhibitory activity of the extracts of lotus flowers (the flower buds of Nelumbo nucifera), has been developed using liquid chromatography-mass spectrometry. The optimum conditions for separation and detection of these 10 alkaloids were achieved on a πNAP column, a reversed-phase column with naphthylethyl group-bonded silica packing material, with CH3CN–0.2% aqueous acetic acid as the mobile phase and using mass spectrometry equipped with a positive-mode electrospray ionization source. According to the protocol established, distributions of these 10 alkaloids in the petal, receptacle, and stamen parts, which were separated from the whole flower, were examined. As expected, excellent correlations were observed between the total alkaloid content and melanogenesis inhibitory activity. Among the active alkaloids, nornuciferine (2) was found to give a carbamate salt (2′′) via formation of an unstable carbamic acid (2′) by absorption of carbon dioxide from the air.
Keywords: lotus flower, Nelumbo nucifera, melanogenesis inhibitor, nuciferine, nornuciferine, quantitative analysis, carbamate salt
1. Introduction
A Nymphaeaceae plant Nelumbo nucifera Gaertn. (common name “lotus” in English) is extensively cultivated in Eastern Asian countries [1,2,3]. All parts of this plant, including the leaves, stamens, flowers, seeds, and rhizomes, have been used as traditional medicines or vegetables for thousands of years [2,3,4]. The lotus flower, the flower buds of N. nucifera, has been used for the treatment of vomiting blood, bleeding caused by internal and external injuries, and various skin diseases, and also as a sedative and an anti-inflammatory agent in traditional Asian medicines [2]. In the course of our studies on the bioactive constituents from the flower buds of N. nucifera, we have isolated several alkaloids, e.g., nuciferine (1), nornuciferine (2), N-methylasimilobine (3), asimilobine (4), pronuciferine (5), and armepavine (6), with melanogenesis inhibitory activities in theophylline-stimulated murine B16 melanoma 4A5 cells [2]. As a result of the increasing interest in lotus flower as a possible cosmetic for skin whitening, there is a strong demand for efficient quality control measurements to ensure the authenticity and content of the active constituents in such products, and to verify the labeled claims. In this paper, we propose a simple, rapid, and precise analytical method for liquid chromatography-mass spectrometry (LC-MS) simultaneous quantitative determination of five aporphine alkaloids (1–5) and five benzylisoquinoline alkaloids, (6), norarmepavine (7), N-methylcoclaurine (8), coclaurine (9), and norjuziphine (10), using a one-step sample preparation procedure.
2. Results and Discussion
2.1. Isolation of Principal Alkaloids (1–10) from Lotus Flower
To obtain the principal alkaloids (1–10), an isolation procedure from this plant material was newly developed in this study by modifying the previously reported method [2]. Thus, dried flower buds of N. nucifera were extracted with methanol under reflux to obtain a methanol extract (9.22% from the dried material). The methanol extract was partitioned into a mixture of EtOAc and 3% aqueous tartaric acid (1:1, v/v) to furnish an acidic EtOAc-soluble fraction (2.88%) and an acidic aqueous solution. The pH of the aqueous solution was adjusted to 9 with saturated aqueous Na2CO3 and then extracted with CHCl3 to obtain a CHCl3-soluble fraction (0.97%). The aqueous layer was further extracted with n-BuOH to obtain an n-BuOH-soluble fraction (0.62%). As shown in Table 1, the methanol extract was found to inhibit theophylline-stimulated melanogenesis (IC50 = 5.6 µg/mL) without cytotoxicity (cell viability in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay: 103.3 ± 7.1%) at 100 µg/mL. Through bioassay-guided separation, the CHCl3-soluble fraction (IC50 = 0.37 µg/mL) was found to be more active than the EtOAc n-BuOH-soluble fractions (IC50 = 11.1 and 13.7 µg/mL, respectively).
Table 1.
Inhibitory effects of the methanol extract and its fractions from lotus flower on theophylline-stimulated melanogenesis and viability in B16 4A5 cells.
| Treatment a | Inhibition (%) | IC50 (µg/mL) |
||||
|---|---|---|---|---|---|---|
| 0 µg/mL | 3 µg/mL | 10 µg/mL | 30 µg/mL | 100 µg/mL | ||
| MeOH ext. | 0.0 ± 1.3 (100.0 ± 9.3) |
28.3 ± 3.7 (110.9 ± 2.7) |
68.9 ± 3.1 ** (124.2 ± 5.1) |
96.4 ± 3.1 ** (126.5 ± 5.3) |
97.0 ± 3.3 ** (103.3 ± 7.1) |
5.6 |
| EtOAc-soluble fraction | 0.0 ± 6.1 (100.0 ± 7.2) |
11.0 ± 1.7 (99.3 ± 9.8) |
51.5 ± 5.3 ** (106.8 ± 5.1) |
83.0 ± 2.9 ** (110.9 ± 4.2) |
100.9 ± 3.4 ** (118.3 ± 10.4) |
11.1 |
| n-BuOH-soluble fraction | 0.0 ± 13.3 (100.0 ± 11.2) |
17.6 ± 7.0 (104.6 ± 11.6) |
35.9 ± 7.1 (104.9 ± 2.6) |
72.3 ± 4.7 ** (114.8 ± 13.9) |
94.7 ± 3.5 ** (104.3 ± 3.8) |
13.7 |
| Treatment | Inhibition (%) |
IC50 (µg/mL) |
||||
| 0 µg/mL | 0.1 µg/mL | 0.3 µg/mL | 1 µg/mL | 3 µg/mL | ||
| CHCl3-soluble fraction | 0.0 ± 1.7 (100.0 ± 3.5) |
19.8 ± 6.8 (101.5 ± 6.1) |
35.6 ± 6.6 ** (103.1 ± 6.1) |
79.2 ± 2.3 ** (114.7 ± 4.6) |
107.2 ± 3.0 ** (107.4 ± 9.5) |
0.37 |
Each value represents the mean ± S.E.M. (n = 4); asterisks denote significant differences from the control group, ** p < 0.01; a Bioassay-guided separation study was carried out using the flower buds of N. nucifera originating in Thailand (NN-1).
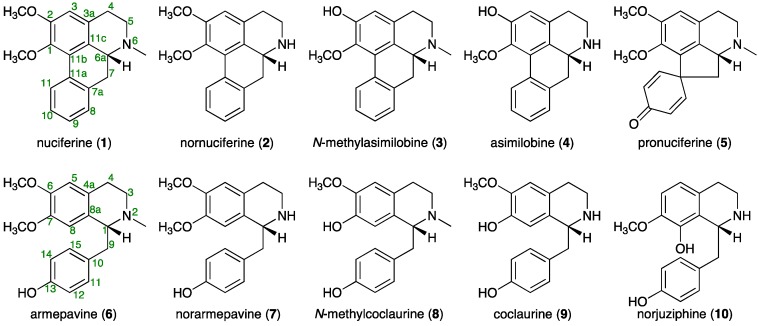
The active CHCl3-soluble fraction was subjected to normal-phase silica gel, reversed-phase ODS column chromatography, and finally HPLC to furnish nuciferine (1, 0.1028%) [2,5,6], nornuciferine (2, 0.0821%) [2,5,6], N-methylasimilobine (3, 0.0094%) [2,7], asimilobine (4, 0.0345%) [2,6,8], pronuciferine (5, 0.0195%) [2,9], armepavine (6, 0.0170%) [2,10], norarmepavine (7, 0.0616%) [10,11], N-methylcoclaurine (8, 0.0053%) [12], coclaurine (9, 0.0042%) [10,13], and norjuziphine (10, 0003%) [14] (Figure 1).
Figure 1.
Aporphine and benzylisoquinoline alkaloids (1–10) from lotus flower.
2.2. Simultaneous Quantitative Analysis of 10 Alkaloids (1–10) in Lotus Flowers
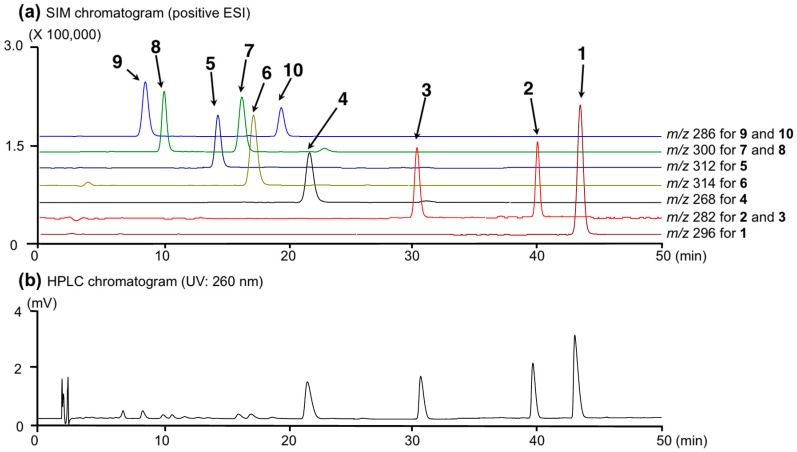
To provide sufficient purity for quantitative analysis, the hydrochlorides of these alkaloids (1–10) were prepared by reported method [10]. As shown in Figure 2, typical LC-MS chromatograms for a standard solution mixture under UV (260 nm) and MS detections by electrospray ionization (ESI) MS under the positive mode demonstrated good baseline separation for all peaks. Each peak was observed at the following retention time and quasimolecular ion peak ([M + H]+) (tR: 1 (43.1 min, m/z 296), 2 (39.5 min, m/z 282), 3 (29.7 min, m/z 282), 4 (21.3 min, m/z 268), 5 (13.9 min, m/z 312), 6 (16.9 min, m/z 314), 7 (15.9 min, m/z 300), 8 (9.9 min, m/z 300), 9 (8.3 min, m/z 286), and 10 (18.8 min, m/z 286)). These peaks were unambiguously assigned by comparison of their retention times with those of authentic specimens [2].
Figure 2.
A typical LC-MS chromatogram of a standard solution mixture (each 10 µg/mL) of alkaloids (1–10). (a) SIM chromatogram (positive ESI); (b) HPLC chromatogram (UV: 260 nm).
Prior to analysis, extraction conditions were examined to optimize the extracts′ quality in association with the contents of the alkaloids (1–10). The extraction efficacies were compared for three solvent systems (methanol, 50% aqueous methanol, and water) under two different conditions (reflux for 120 min or sonication for 30 min, each twice). As shown in Table 2, “reflux in methanol” afforded the highest contents of the active alkaloids (1–10). Therefore, all the analytical samples were prepared by employing the method “reflux in methanol for 120 min”.
Table 2.
Extraction efficiently of alkaloids (1–10) from lotus flower.
| Extraction Method | Extraction Yield (%) | Contents (mg/g in Dry Material) a | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| Methanol, reflux | 15.0 | 1.76 (100) | 1.75 (100) | 0.07 (100) | 0.63 (100) | 0.69 (100) | 0.83 (100) | 1.45 (100) | 5.73 (100) | 1.30 (100) | 0.75 (100) | 14.96 (100) |
| 50% Methanol, reflux | 25.3 | 1.09 (62) | 1.35 (77) | 0.05 (71) | 0.50 (79) | 0.61 (88) | 0.78 (94) | 1.35 (93) | 3.79 (66) | 0.94 (73) | 0.56 (75) | 11.02 (74) |
| H2O, reflux | 23.1 | 0.24 (14) | 0.35 (20) | n.d. b | 0.21 (33) | 0.18 (26) | 0.38 (45) | 0.78 (54) | 2.57 (45) | 0.66 (51) | 0.29 (38) | 5.66 (38) |
| Methanol, sonication | 9.6 | 0.88 (50) | 1.11 (64) | 0.03 (44) | 0.39 (62) | 0.33 (48) | 0.47 (56) | 0.97 (67) | 2.77 (48) | 0.70 (54) | 0.42 (56) | 8.07 (54) |
| 50% Methanol, sonication | 22.0 | 0.98 (56) | 1.27 (73) | 0.04 (58) | 0.49 (78) | 0.47 (69) | 0.80 (96) | 1.38 (95) | 3.93 (69) | 0.97 (75) | 0.59 (79) | 10.92 (73) |
| H2O, sonication | 19.3 | 0.14 (8) | 0.21 (12) | n.d. b | 0.12 (20) | 0.08 (11) | 0.25 (30) | 0.53 (37) | 1.91 (33) | 0.48 (37) | 0.19 (26) | 3.91 (26) |
Extraction efficiently was tested using NN-1 (loss of drying 10.33%); a relative value (%) against the content obtained by methanol under reflux is given in parentheses; b less than the quantitation limit.
Some analytical parameters, such as linearity and limit of quantitation of the developed method, were evaluated as shown in Table 3. The calibration curve was linear in the range studied (0.5–50 µg/mL) showing a correlation coefficient (R2) of greater than 0.9996 for each constituent. Linear regression equations of their calibration curves for each constituent are described in Table 3, where y is the peak area and x is the concentration of the analyte. The detection and quantitation limits were estimated to be 0.17–0.90 and 0.51–2.65 ng, respectively, indicating sufficient sensitivity of this method. The relative standard deviation (RSD) values were 0.25%–1.36% for intra-day and 0.39%–1.40% for inter-day assays. Accuracy was determined in recovery experiments using the methanol extract of NN-1. As shown in Table 4, recovery rates of 92.3%–105.8% were obtained, with RSD values of lower than 1.6%.
Table 3.
Linearities, detection and quantitation limits, and precisions for alkaloids (1–10) in lotus flower.
| Analyte | Regression Equation a | Correlation Coefficient | Detection Limit b (ng) | Quantitation Limit b (ng) | Precision c (RSD, %) | |
|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | |||||
| Nuciferine (1) | y = 7477635x − 1302 | 0.9998 | 0.17 | 0.51 | 0.25 | 0.59 |
| Nornuciferine (2) | y = 2698708x − 10941 | 1.0000 | 0.71 | 2.16 | 0.79 | 0.43 |
| N-Methylasimilobine (3) | y = 7054297x + 243961 | 0.9996 | 0.32 | 0.99 | 1.36 | 1.40 |
| Asimilobine (4) | y = 2076494x − 36021 | 0.9999 | 0.70 | 2.13 | 0.63 | 0.57 |
| Pronuciferine (5) | y = 3522995x + 101328 | 0.9998 | 0.73 | 2.18 | 0.95 | 1.08 |
| Armepavine (6) | y = 2076494x − 36021 | 0.9999 | 0.32 | 0.97 | 0.68 | 1.10 |
| Norarmepavine (7) | y = 1998354x − 15296 | 0.9999 | 0.81 | 2.47 | 0.54 | 0.73 |
| N-Methylcoclaurine (8) | y = 1595194x + 53314 | 0.9999 | 0.90 | 2.71 | 0.59 | 0.86 |
| Coclaurine (9) | y = 1878370x + 16838 | 0.9999 | 0.44 | 1.33 | 0.98 | 0.39 |
| Norjuziphine (10) | y = 1745634x + 15240 | 1.0000 | 0.88 | 2.65 | 0.64 | 0.66 |
a In the regression equation, x is the concentration of the analyte solution (µg/mL), and y is the peak area of the analyte; b values are the amount of the analyte injected on-column and c precision of the analytical method were tested using the methanol extract of NN-1 (n = 5).
Table 4.
Recoveries for alkaloids (1–10) from lotus flower.
| Add (µg/mL) | Recovery a (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 10 | 98.7 ± 0.6 | 101.4 ± 0.7 | 95.3 ± 1.0 | 99.0 ± 0.9 | 93.2 ± 0.7 | 98.5 ± 0.3 | 104.0 ± 0.7 | 94.7 ± 1.4 | 97.4 ± 0.2 | 97.4 ± 1.0 |
| 15 | 92.3 ± 0.4 | 101.7 ± 0.2 | 97.5 ± 0.7 | 101.8 ± 0.1 | 98.1 ± 0.5 | 102.2 ± 0.6 | 99.4 ± 1.1 | 99.8 ± 1.6 | 104.0 ± 0.2 | 100.2 ± 0.8 |
| 20 | 98.4 ± 0.1 | 105.8 ± 0.7 | 101.2 ± 0.8 | 102.0 ± 0.7 | 95.9 ± 0.8 | 105.1 ± 0.6 | 99.9 ± 0.9 | 96.3 ± 1.1 | 105.8 ± 0.3 | 95.3 ± 0.8 |
a The recovery rates were determined by adding analytes of three different concentrations (10, 15, and 20 µg/mL) to the sample solution; recoveries spiked with the methanol extract of NN-1 (each 400 µg/mL, n = 3).
According to the protocol thus established, contents of the alkaloids (1–10) collected in two different regions (NN-1 in Thailand; NN-5 in Taiwan) were measured. The assay was found to be reproducible, precise, and readily applicable to the quality evaluation of lotus flower′s extracts. As shown in Table 5, N-methylcoclaurine (8, NN-1: 5.73 mg/g in dry material; NN-5: 2.88 mg/g) was the richest constituent among the alkaloids (1–10). The total alkaloid content in the Thai (NN-1: 14.96 mg/g) and Taiwanese (NN-5: 3.53 mg/g) samples were quite different. However, a more extensive study would be required to confirm that this result was due to differences between regions. To characterize the distribution of the alkaloids (1–10) in the flower, the whole flower parts (NN-1 and NN-5) were separated into petals (NN-2 and NN-6), receptacles (NN-3 and NN-7), and stamens (NN-4 and NN-8); then, quantitative analysis of each separated sample was performed. It was found that the alkaloids (1–10) were mainly contained in the petal part. Furthermore, other parts of the lotus plant (e.g., leaf (NN-9), fruit (NN-10 and 11), and embryo parts (NN-12), which are used for traditional medicines) were also examined. It was found that the total alkaloid content of the leaf (NN-9: 1.20 mg/g), fruit (NN-10 and NN-11: each less than the quantitation limit), and embryo parts (NN-12: 0.64 mg/g) of N. nucifera were lower than those of the flower buds (NN-1 and NN-5) (Table S1).