Table 5.
Contents of alkaloids (1–10) in the methanol extracts from lotus flower.
| Sample No. | Part | Loss of Drying a (%) | Extraction Yield b (%) | Contents (mg/g in Dry Material) a | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||
| NN-1 | whole flowers | 10.3 | 15.0 | 1.76 | 1.75 | 0.07 | 0.63 | 0.69 | 0.83 | 1.45 | 5.73 | 1.30 | 0.75 | 14.96 |
| NN-2 | petals | 8.6 | 16.6 | 1.99 | 2.41 | 0.07 | 1.22 | 0.62 | 1.02 | 1.74 | 5.85 | 1.60 | 0.52 | 17.04 |
| NN-3 | receptacles | 10.0 | 8.6 | 0.06 | 0.07 | n.d. c | 0.01 | 0.01 | 0.01 | 0.01 | 0.20 | 0.04 | n.d. c | 0.41 |
| NN-4 | stamens | 8.6 | 20.0 | 0.58 | 0.68 | n.d. c | 0.25 | 0.23 | 0.47 | 0.64 | 2.88 | 0.60 | 0.32 | 6.65 |
| NN-5 | whole flowers | 8.1 | 16.3 | 0.56 | 0.27 | n.d. c | 0.04 | n.d. c | 0.23 | 0.34 | 1.74 | 0.19 | 0.16 | 3.53 |
| NN-6 | petals | 7.3 | 19.0 | 0.80 | 0.34 | n.d. c | n.d. c | 0.01 | 0.36 | 0.52 | 3.14 | 0.32 | 0.27 | 5.76 |
| NN-7 | receptacles | 9.2 | 7.4 | 0.27 | 0.53 | n.d. c | n.d. c | 0.05 | 0.03 | 0.03 | 0.74 | 0.27 | n.d.c | 1.92 |
| NN-8 | stamens | 7.9 | 15.5 | 0.01 | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | 0.03 | 0.01 | 0.03 | 0.08 |
a Each powdered sample was dried at 105 °C for 8 h; b each powdered sample was extracted two times with methanol under reflux for 120 min and c less than the quantitation limit.
2.3. Ammonium Carbamate Salt (2′′) Formation from the Free Alkaloid (2)
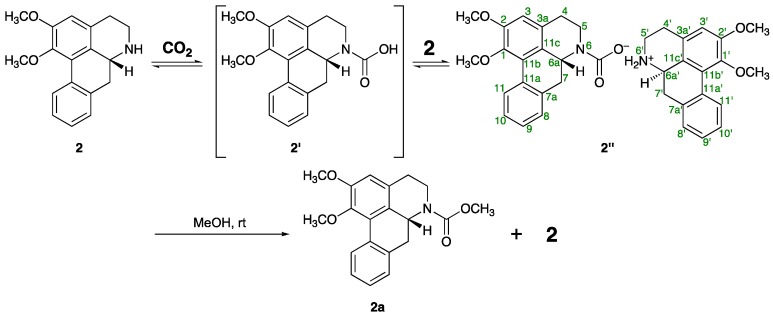
The gradual transformation of one of the alkaloids isolated in this study, nornuciferine (2), into a highly polar material 2′′ was observed, when 2 was exposed to the atmosphere in deuterated chloroform (CDCl3) at room temperature. After three weeks of standing in CDCl3, compound 2′′ was obtained as a main product (Figure 3). As summarized in Table 6, 1H- and 13C-NMR spectra of 2′′ suggested that there are two kinds of parts derived from the nornuciferine framework in the structure of 2′′. Thus, with respect to one of the nornuciferine parts, five carbons α and β to the nitrogen atom appeared as pair signals [δC: 29.9/30.2 (C-4), 41.8/44.4 (C-5), 54.9/55.8 (C-6a), 33.9/35.9 (C-7), 124.8/124.9 (C-11c)] in the 13C-NMR spectrum of 2′. Additionally, a pair of signals, which corresponded to an amide type carbonyl carbon, was also observed at δC 157.5 and 160.1. The chemical shift of the signals suggested that the nitrogen atom of 2 was functionalized as a carbamate anion by the CO2 uptake from the atmosphere. On the other hand, in the 1H-NMR spectrum of 2′′ a downfield shift owing to the ammonium ion formation was observed with respect to the signals due to C-5′ methylene (at δH 3.24 and 3.87) and C-6a′ methine (at δH 4.29) protons of the other nornuciferine parts as compared with those of 2 [δH: 3.01 and 3.40 (H2-5), 3.85 (H-6a)]. Two broad singlets, which appeared at the highly-deshielded regions (δH 9.96 and 10.84), were due to acidic protons, which also support the ammonium ion structure depicted in Figure 3. The anticipated structure of 2′ was strongly supported by the IR spectrum, which showed N+–H and C=O stretching absorptions at 2720–2500 and 1721 cm−1, respectively. Moreover, the positive ion part of 2′′ was detected as a Na adduct ion signal at m/z 282.1483 [M − C19H18NO4]+ (calcd for C18H20O2, 282.1489) in the positive ESI mode; however, a signal due to the carbamate group was not detected in the negative ESI mode. Fortunately, the negative ion part of 2′′ could be confirmed as the corresponding methyl carbamate 2a. Thus, 2′′ easily gave a 1:1 mixture of methyl carbamate 2a and original amine 2 by treatment with methanol at room temperature (Figure S1). As shown in Table 6, compound 2a showed similar 13C-NMR spectroscopic properties to those of 2′′ and/or 2, except for a singlet (δC 52.6) due to the methyl carbon of the NCO2CH3 moiety, which was confirmed by the correlation between the singlet at δH 3.76 due to the methyl protons and a singlet at δC 156.0 due to carbonyl carbon in the HMBC of 2a. In the positive ESIMS of 2a, a quasimolecular ion peak was observed at m/z 362.1361 [M + Na]+ (calced for C20H21NO4Na, 362.1363).
Figure 3.
Chemical transformation of nornuciferine (2) into its ammonium carbamate salt (2′′) and to methyl carbamate (2a).
Table 6.
1H- (800 MHz) and 13C- (200 MHz) NMR data for 2′′, 2a, and original alkaloid 2 in CDCl3.
| Position | 2′′ (anion part) | Position | 2′′ (cation part) | ||
|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | ||
| 1 | 146.0 a | 1′ | 146.2 a | ||
| 2 | 152.5 b | 2′ | 153.7 b | ||
| 3 | 6.69 (s) | 111.4 | 3′ | 6.67 (s) | 111.4 |
| 3a | 128.7 | 3a′ | 125.7 | ||
| 4 | 2.74 (br d, ca. 15) 2.98 (br d, ca. 15) |
29.9, 30.2 | 4′ | 2.95 (dd, 3.8, 16.8) 3.62 (ddd, 5.7, 13.5, 16.8) |
25.5 |
| 5 | 3.20–3.35 (m) 4.62 (br d, ca. 12.5) |
41.8, 44.4 | 5′ | 3.24 (br ddd-like, ca. 13.5, 13.5, 13.5) 3.87 (br dd-like, ca. 5.7, 13.5) |
41.4 |
| 6a | 4.92 (br d, ca. 13) | 54.9, 55.8 | 6a′ | 4.29 (br dd-like, ca. 13.5, 13.5) | 53.0 |
| 7 | 2.86–2.90 (m) 3.02–3.14 (m) |
33.9, 35.9 | 7′ | 3.38 (dd, 13.5, 13.5) 3.45 (dd, 4.5, 13.5) |
34.0 |
| 7a | 135.7 | 7a′ | 133.1 | ||
| 8 | 7.24–7.30 (m) | 128.0 c | 8′ | 7.24–7.30 (m) | 128.3 c |
| 9 | 7.24–7.30 (m) | 127.4 c | 9′ | 7.24–7.30 (m) | 128.2 c |
| 10 | 7.35 (m) | 127.3 c | 10′ | 7.35 (m) | 127.8 c |
| 11 | 8.44 (br s-like) | 128.5 c | 11′ | 8.41 (d, 7.9) | 128.6 c |
| 11a | 131.3 | 11a′ | 131.3 | ||
| 11b | 127.5 d | 11b′ | 127.0 d | ||
| 11c | 124.8, 124.9 | 11c′ | 121.4 | ||
| 1-OCH3 | 3.667 e (s) | 60.3 f | 1′-OCH3 | 3.673 e (s) | 60.0 f |
| 2-OCH3 | 3.90 g (s) | 56.0 h | 2′-OCH3 | 3.91 g (s) | 55.9 h |
| N-COO | 157.5, 160.1 | NH2 | 9.96 (br ddd-like, ca. 13.5, 13.5, 13.5 ) 10.84 (br d-like, ca. 13.5) |
||
| Position | 2a | Position | 2 | ||
| δH (J in Hz) | δC | δH (J in Hz) | δC | ||
| 1 | 145.7 | 1 | 145.3 | ||
| 2 | 152.1 | 2 | 152.2 | ||
| 3 | 6.67 (s) | 111.5 | 3 | 6.65 (s) | 111.8 |
| 3a | 126.1 | 3a | 128.5 | ||
| 4 | 2.65 (br d, ca. 15) 2.88 (m) |
30.3 | 4 | 2.71 (d, 13.1) 3.05 (m) |
28.9 |
| 5 | 3.00 (br dd, ca. 11, 13) 4.73 (br d, ca. 13) |
38.8 | 5 | 3.01 (m) 3.40 (br q, ca. 6) |
43.0 |
| 6a | 4.46 (br s) | 51.4 | 6a | 3.85 (br dd, ca. 5, 14) | 53.5 |
| 7 | 2.86 (m) 2.98 (dd, 12.8, 15.8) |
35.2 | 7 | 2.77 (t, 13.8) 2.87 (dd, 4.6, 13.8) |
37.3 |
| 7a | 136.8 | 7a | 135.9 | ||
| 8 | 7.25 (dd, 1.6, 7.8) | 128.2 | 8 | 7.24 (m) | 127.8 |
| 9 | 7.27 (br dd, ca. 8, 8) | 127.5 | 9 | 7.21 (ddd, 1.1, 7.1, 7.1) | 127.4 |
| 10 | 7.32 (ddd, 1.6, 7.8, 8.0) | 126.9 | 10 | 7.30 (m) | 127.0 |
| 11 | 8.44 (br d, ca. 8) | 128.3 | 11 | 8.39 (br d, ca. 8) | 128.4 |
| 11a | 131.7 | 11a | 132.1 | ||
| 11b | 127.6 | 11b | 126.5 | ||
| 11c | 129.7 | 11c | 128.7 | ||
| 1-OCH3 | 3.66 (s) | 60.2 | 1-OCH3 | 3.67 (s) | 60.2 |
| 2-OCH3 | 3.90 (s) | 56.0 | 2-OCH3 | 3.88 (s) | 55.9 |
| N-COO | 156.0 | ||||
| N-CO2CH3 | 3.76 (s) | 52.6 | |||
a–h May be interchangeable.
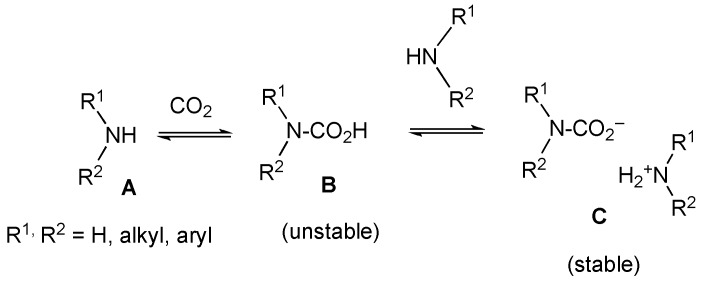
It is well known that ammonia, primary amines, or secondary amines (A) absorb CO2 to transform into the corresponding carbamic acids (B), which easily react with the original amines to produce stable carbamic acid ammonium salts and (C) as shown in Figure 4 [15,16,17,18,19,20,21,22,23,24]. Therefore, it is reasonable to anticipate that the product 2′′ forms via an acid-base reaction between original amine 2 and an unstable carbamic acid (2′), which was obtained by the CO2 absorption reaction with the nitrogen atom of 2.
Figure 4.
The reported reactivity of amines with CO2.
2.4. Effects of the Hydrochlorides of These Alkaloids (1–10) and 2a on Theophylline-Stimulated Melanogenesis Inhibitory Activity
To clarify the efficacy of the established quantitative analysis of 10 alkaloids (1–10) as a quality control for lotus flower, correlations between the total alkaloid contents and the melanogenesis inhibitory activities of the corresponding extracts were examined. Previously, we have reported the melanogenesis inhibitory activities of free nuciferine (1, IC50 = 15.8 µM), nornuciferine (2, 62.9 µM), N-methylasimilobine (3, 14.5 µM), asimilobine (4, >100 µM), pronuciferine (5, 47.9 µM), and armepavine (6, 25.6 µM) [2]. Since the hydrochlorides of these alkaloids (1–10), which were of higher purity and stability than those of the corresponding free alkaloids, were used for the standard samples of the present quantitative analysis, the melanogenesis inhibitory activities of these hydrochloride salts were newly examined. It was found that aporphine alkaloids, nuciferine (1, IC50 = 7.1 µM) and nornuciferine (2, 3.9 µM), and benzylisoquinoline alkaloids, armepavine (6, 6.5 µM), norarmepavine (7, 7.5 µM), N-methylcoclaurine (8, 6.5 µM), and coclaurine (9, 3.9 µM), were found to show relatively strong inhibitory activities without notable cytotoxic effects at the effective concentration (Table 7). These alkaloids were more potent than arbutin (IC50 = 174 µM), a commercially used melanogenesis inhibitor used as a positive control [25,26,27]. Among them, 2 and 9 showed especially strong activity, which showed more than 40 times greater than that of arubutin. Previously, the naturally-occurring aporphine alkaloid, 4,5-didehydroguadiscine (IC50 = 4.7 µM) isolated from Hornschuchia oblique, has been reported [28]. To the best of our knowledge, compounds 2 and 9 are the most potent melanogenesis inhibitors within this class of natural products.
Table 7.
Inhibitory effects of the alkaloids (1–10) and 2a on theophylline-stimulated melanogenesis and viability in B16 4A5 cells.
| Treatment | Inhibition (%) | IC50 | |||||
|---|---|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | (µM) | (µg/mL) b | |
| Nuciferine·HCl (1) a | 0.0 ± 4.0 (100.0 ± 2.6) |
28.3 ± 4.2 ** (112.1 ± 2.6) |
57.8 ± 2.3 ** (112.1 ± 2.5) |
89.7 ± 1.7 ** (100.9 ± 5.8) |
91.8 ± 2.6 ** (45.9 ± 3.3 #) |
7.1 | 2.4 |
| Nornuciferine·HCl (2) a | 0.0 ± 3.4 (100.0 ± 4.9) |
44.6 ± 3.4 ** (99.5 ± 8.2) |
70.6 ± 3.6 ** (93.9 ± 4.1) |
94.3 ± 3.2 ** (70.6 ± 4.8 #) |
— (1.9 ± 0.1 #) |
3.9 | 1.2 |
| N-Methylasimilobine·HCl (3) a | 0.0 ± 4.7 (100.0 ± 3.9) |
3.3 ± 2.9 (96.6 ± 2.6) |
19.5 ± 2.4 ** (100.4 ± 3.0) |
63.4 ± 5.2 ** (119.3 ± 9.4) |
89.8 ± 2.1 ** (57.7 ± 1.3 #) |
43.1 | 13.7 |
| Asimilobine·HCl (4) a | 0.0 ± 8.1 (100.0 ± 5.7) |
16.2 ± 12.8 (96.5 ± 7.5) |
31.9 ± 2.2 ** (98.9 ± 10.0) |
87.2 ± 3.3 ** (76.6 ± 6.3) |
— (13.7 ± 2.2 #) |
11.3 | 3.4 |
| Pronuciferine·HCl (5) a | 0.0 ± 10.7 (100.0 ± 4.5) |
23.1 ± 3.4 (104.2 ± 3.4) |
18.8 ± 1.2 (97.4 ± 0.7) |
37.2 ± 2.6 ** (98.4 ± 1.8) |
88.4 ± 3.7 ** (89.1 ± 9.4) |
47.1 | 16.3 |
| Armepavine·HCl (6) a | 0.0 ± 6.0 (100.0 ± 2.5) |
33.9 ± 0.8 ** (104.0 ± 3.3) |
58.5 ± 7.1 ** (104.0 ± 7.7) |
81.8 ± 3.0 ** (104.0 ± 7.2) |
97.4 ± 0.4 ** (78.1 ± 2.0) |
6.5 | 3.4 |
| Norarmepavine·HCl (7) a | 0.0 ± 3.1 (100.0 ± 2.6) |
32.6 ± 4.4 ** (86.4 ± 4.0) |
53.5 ± 8.5 ** (81.2 ± 4.0) |
81.6 ± 1.4 ** (83.3 ± 2.2) |
90.5 ± 1.2 ** (68.8 ± 1.4 #) |
7.5 | 2.5 |
| N-Methylcoclaurine·HCl (8) a | 0.0 ± 5.4 (100.0 ± 3.0) |
38.6 ± 2.4 ** (97.1 ± 1.5) |
55.7 ± 3.4 ** (92.8 ± 4.1) |
74.7 ± 2.0 ** (96.4 ± 4.2) |
— — |
6.5 | 2.2 |
| Coclaurine·HCl (9) a | 0.0 ± 2.9 (100.0 ± 2.9) |
45.6 ± 7.7 ** (97.0 ± 7.7) |
65.4 ± 2.5 ** (96.2 ± 5.0) |
82.4 ± 3.5 ** (86.5 ± 7.7) |
68.0 ± 6.4 ** (53.1 ± 8.3 #) |
3.9 | 1.3 |
| Norjuziphine·HCl (10) a | 0.0 ± 5.4 (100.0 ± 5.3) |
18.5 ± 4.0 * (98.9 ± 6.0) |
36.8 ± 4.5 ** (91.3 ± 4.9) |
94.4 ± 2.0 ** (83.9 ± 8.3) |
106.0 ± 2.0 ** (57.0 ± 2.4 #) |
14.4 | 4.6 |
| 2a | 0.0 ± 7.3 (100.0 ± 4.7) |
13.1 ± 8.9 (109.8 ± 4.3) |
43.1 ± 7.8 ** (127.5 ± 4.8) |
54.5 ± 4.3 ** (129.1 ± 2.8) |
— — |
19.9 | 6.7 |
| Treatment | Inhibition (%) | IC50 | |||||
| 0 µM | 30 µM | 100 µM | 300 µM | 1000 µM | (µM) | (µg/mL) | |
| Arbutin [25,26,27] | 0.0 ± 1.4 (100.0 ± 2.1) |
20.4 ± 0.5 (82.4 ± 3.0) |
38.1 ± 0.9 ** (78.1 ± 1.9) |
61.5 ± 0.6 ** (79.8 ± 2.2) |
83.7 ± 0.5 ** (53.1 ± 1.8 #) |
174 | 47.4 |
Each value represents the mean ± S.E.M. (n = 4); asterisks denote significant differences from the control group, * p < 0.05, ** p < 0.01; # cytotoxic effects were observed, and values in parentheses indicate cell viability (%) in MTT assay; commercial arubutin was purchased from Nakalai Tesque Inc., (Kyoto, Japan); a each alkaloid was evaluated by its hydrochloride salt; b each IC50 value was converted to µg/mL of corresponding free alkaloid.
2.5. Effects on Mushroom Tyrosinase
To characterize the mode of action of melanogenesis inhibitory activities of the alkaloids, inhibitory effects on (i) enzymatic tyrosinase activity and (ii) expressions of tyrosinase-related proteins (TRPs) e.g. tyrosinase, TRP-1, and TRP-2 were examined.
A copper-containing enzyme tyrosinase is a key enzyme in melanin biosynthesis involved in determining the color of skin and hair. It catalyzes oxidation of both l-tyrosine and l-DOPA, following another oxidation of l-DOPA to dopaquinone and, finally, oxidative polymerization via several dopaquinone derivatives to yield melanin. Tyrosinase inhibitors are being clinically used for the treatment of several dermatological disorders associated with melanin hyperpigmentation. The tyrosinase inhibitor kojic acid is commonly used as an additive in cosmetics for skin whitening and/or depigmentation [25,26,27]. As shown in Table 8, none of the alkaloids showed inhibitory activities when using both l-tyrosine and l-DOPA as substrates. This suggests that tyrosinase inhibition is barely involved in the mechanisms of action of these melanogenesis inhibitors.
Table 8.
Effects on activity of tyrosinase from mushroom.
| Substrate:Treatment | Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| l-Tyrosine | l-DOPA | |||||
| 0 µM | 10 µM | 100 µM | 0 µM | 10 µM | 100 µM | |
| Nuciferine·HCl (1) a | 0.0 ± 3.2 | 6.5 ± 3.3 | 30.4 ± 1.9 ** | 0.0 ± 2.9 | 4.4 ± 3.8 | 11.0 ± 3.4 |
| Nornuciferine·HCl (2) a | 0.0 ± 2.6 | 1.0 ± 0.7 | 14.2 ± 1.5 ** | 0.0 ± 3.1 | 15.4 ± 4.1 | 8.7 ± 4.1 |
| N-Methylasimilobine·HCl (3) a | 0.0 ± 3.6 | 7.2 ± 7.3 | 0.8 ± 3.5 | 0.0 ± 1.5 | 2.3 ± 1.5 | 3.1 ± 3.9 |
| Asimilobine·HCl (4) a | 0.0 ± 1.8 | 10.5 ± 2.5 * | 14.0 ± 1.2 ** | 0.0 ± 3.3 | 3.2 ± 2.4 | 5.7 ± 3.0 |
| Pronuciferine·HCl (5) a | 0.0 ± 5.3 | −0.4 ± 2.4 | −4.4 ± 8.1 | 0.0 ± 2.0 | 6.5 ± 2.0 | 5.3 ± 5.2 |
| Armepavine·HCl (6) a | 0.0 ± 4.4 | −1.9 ± 0.9 | 40.2 ± 3.7 ** | 0.0 ± 0.5 | 4.5 ± 0.8 | 2.9 ± 1.2 |
| Norarmepavine·HCl (7) a | 0.0 ± 1.8 | −1.7 ± 1.5 | 23.3 ± 1.6 ** | 0.0 ± 2.7 | −1.3 ± 1.4 | 1.5 ± 1.5 |
| N-Methylcoclaurine·HCl (8) a | 0.0 ± 5.2 | −5.0 ± 5.7 | 15.3 ± 4.3 | 0.0 ± 2.3 | 6.1 ± 1.4 | 7.4 ± 0.8 |
| Coclaurine·HCl (9) a | 0.0 ± 2.3 | 9.0 ± 0.7 * | 35.1 ± 1.7 ** | 0.0 ± 2.5 | −4.9 ± 1.1 | 4.4 ± 1.1 |
| Norjuziphine·HCl (10) a | 0.0 ± 2.5 | 5.1 ± 1.1 | 27.4 ± 3.0 ** | 0.0 ± 2.0 | 1.8 ± 0.7 | 22.3 ± 3.6 ** |
| 2a | 0.0 ± 1.5 | 5.3 ± 1.7 | 2.9 ± 0.7 | 0.0 ± 2.7 | 3.2 ± 1.1 | 6.6 ± 2.3 |
| Substrate: l-Tyrosine | Inhibition (%) | |||||
| Treatment | 0 µM | 10 µM | 30 µM | 100 µM | 300 µM | IC50 (µM) |
| Kojic acid [25,26,27] | 0.0 ± 2.4 | 12.2 ± 3.3 | 46.4 ± 2.6 ** | 66.5 ± 2.1 ** | 96.8 ± 0.9 ** | 43.6 |
| Substrate: l-DOPA | Inhibition (%) | |||||
| Treatment | 0 µM | 10 µM | 30 µM | 100 µM | 300 µM | IC50 (µM) |
| Kojic acid [25,26,27] | 0.0 ± 0.9 | 22.3 ± 2.1 ** | 50.6 ± 0.6 ** | 78.2 ± 0.7 ** | 89.3 ± 0.3 ** | 29.6 |
Each value represents the mean ± S.E.M. (n = 4); asterisks denote significant differences from the control group, * p < 0.05, ** p < 0.01; commercial kojic acid was purchased from Nakalai Tesque Inc., (Kyoto, Japan); a each alkaloid was evaluated by its hydrochloride salt.
2.6. Effects on Expression of Tyrosinase, TRP-1, and TRP-2
The TRP enzyme family (tyrosinase, TRP-1, and TRP-2) catalyze the major steps in melanin synthesis [25,26,27]. To clarify the mechanisms of action of these active constituents, we examined the effects of the principal alkaloids (1, 2, 6, 7, and 9) on expression of mRNAs for tyrosinase, TRP-1, and TRP-2 in B16 melanoma 4A5 cells. Except for compound 7, the suppression tendency of mRNA expression for tyrosinase in these alkaloids was observed as presented in Table 9.
Table 9.
Effects of 1, 2, 6, 7, and 9 on expression of tyrosinase, TRP-1, and TRP-2 mRNA in B16 4A5 cells.
| Treatment | Tyrosinase mRNA/-actin mRNA | ||
|---|---|---|---|
| 0 µM | 3 µM | 10 µM | |
| Nuciferine·HCl (1) a | 1.00 ± 0.15 | 0.59 ± 0.03 * | 0.45 ± 0.05 * |
| Nornuciferine·HCl (2) a | 1.00 ± 0.19 | 0.76 ± 0.05 | 0.51 ± 0.10 |
| Armepavine·HCl (6) a | 1.00 ± 0.14 | 0.86 ± 0.13 | 0.74 ± 0.02 |
| Norarmepavine·HCl (7) a | 1.00 ± 0.24 | 0.81 ± 0.08 | 1.00 ± 0.11 |
| Coclaurine·HCl (9) a | 1.00 ± 0.14 | 0.82 ± 0.21 | 0.52 ± 0.05 |
| Treatment | TRP-1 mRNA/-actin mRNA | ||
| 0 µM | 3 µM | 10 µM | |
| Nuciferine·HCl (1) a | 1.00 ± 0.12 | 1.18 ± 0.17 | 1.18 ± 0.25 |
| Nornuciferine·HCl (2) a | 1.00 ± 0.10 | 1.21 ± 0.18 | 1.15 ± 0.19 |
| Armepavine·HCl (6) a | 1.00 ± 0.22 | 0.98 ± 0.32 | 0.83 ± 0.15 |
| Norarmepavine·HCl (7) a | 1.00 ± 0.09 | 0.87 ± 0.22 | 1.03 ± 0.25 |
| Coclaurine·HCl (9) a | 1.00 ± 0.24 | 0.51 ± 0.08 | 0.66 ± 0.13 |
| Treatment | TRP-2 mRNA/-actin mRNA | ||
| 0 µM | 3 µM | 10 µM | |
| Nuciferine·HCl (1) a | 1.00 ± 0.16 | 1.33 ± 0.35 | 0.87 ± 0.16 |
| Nornuciferine·HCl (2) a | 1.00 ± 0.12 | 0.92 ± 0.20 | 1.36 ± 0.09 |
| Armepavine·HCl (6) a | 1.00 ± 0.05 | 1.07 ± 0.22 | 0.81 ± 0.22 |
| Norarmepavine·HCl (7) a | 1.00 ± 0.18 | 0.80 ± 0.24 | 0.77 ± 0.14 |
| Coclaurine·HCl (9) a | 1.00 ± 0.11 | 1.02 ± 0.22 | 1.07 ± 0.07 |
Each value represents the mean ± S.E.M. (n = 3); asterisks denote significant differences from the control group, * p < 0.05; a each alkaloid was evaluated by its hydrochloride salt.
2.7. Correlation between the Melanogenesis Inhibitory Activity and Total Contents of Alkaloids (1–10) in Lotus Flower Extracts
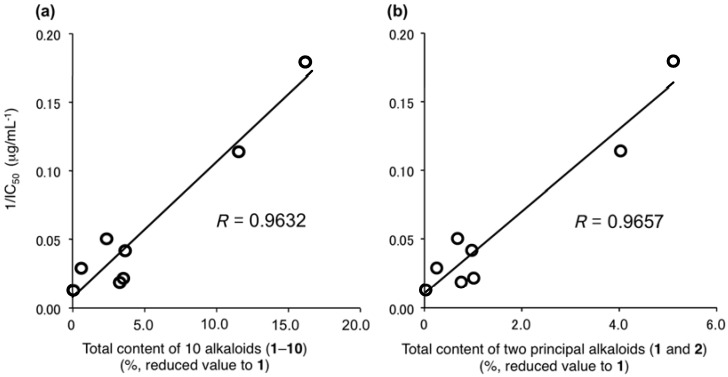
The inhibitory effects of the methanol extracts of the lotus flowers (NN-1–8) on theophylline-stimulated melanogenesis were examined. As a result, the IC50 values were detected in all the lotus flower samples in the ranges of 5.8–78.9 µg/mL (Table 10). In Figure 5, correlations between the total content of 10 alkaloids (value reduced to 1) and the melanogenesis inhibitory activities (1/IC50) of the corresponding extracts were plotted. As expected, excellent correlations were observed between the total content and the inhibitory activities (R = 0.9632). As the minimum involvement, these correlations were shown between the content of two principal alkaloids (1 and 2) and the activities (R = 0.9657). In addition, the methanol extracts from the leaf and fruit parts of N. nucifera (NN-9–11) did not show melanogenesis inhibitory activities (IC50 > 100 µg/mL, Table S2). On the other hand, despite the fact that the methanol extract from the embryo of N. nucifera (NN-12) contained scarcely any alkaloids (1–10) (vide supra), potent melanogenesis inhibitory activity was observed (IC50 = 4.5 µg/mL, Table S2). This evidence suggested that other melanogenesis inhibitory active constituents are included in the embryo part.
Table 10.
Inhibitory effects of the methanol extract from lotus flower (NN-1–NN-8) on theophylline-stimulated melanogenesis and viability in B16 4A5 cells.
| Sample No. | Inhibition (%) | IC50 | ||||
|---|---|---|---|---|---|---|
| 0 µg/mL | 3 µg/mL | 10 µg/mL | 30 µg/mL | 100 µg/mL | (µg/mL) | |
| NN-1 | 0.0 ± 1.3 (100.0 ± 9.3) |
28.3 ± 3.7 (110.9 ± 2.7) |
68.9 ± 3.1 ** (124.2 ± 5.1) |
96.4 ± 3.1 ** (126.5 ± 5.3) |
97.0 ± 3.3 ** (103.3 ± 7.1) |
5.6 |
| NN-2 | 0.0 ± 5.7 (100.0 ± 2.7) |
15.7 ± 8.2 (113.3 ± 2.2) |
49.9 ± 12.0 ** (114.6 ± 1.8) |
108.2 ± 4.1 ** (128.9 ± 2.8) |
103.8 ± 6.7 ** (116.2 ± 2.5) |
8.8 |
| NN-3 | 0.0 ± 5.9 (100.0 ± 6.8) |
18.6 ± 4.2 (104.7 ± 4.3) |
26.1 ± 5.0 ** (102.6 ± 2.9) |
72.9 ± 0.7 ** (119.3 ± 5.4) |
86.8 ± 4.1 ** (108.2 ± 3.6) |
34.7 |
| NN-4 | 0.0 ± 10.0 (100.0 ± 5.3) |
10.7 ± 8.1 (99.4 ± 7.1) |
28.3 ± 4.7 (99.1 ± 11.1) |
95.7 ± 1.6 ** (98.0 ± 5.0) |
107.0 ± 2.1 ** (92.4 ± 6.3) |
24.0 |
| NN-5 | 0.0 ± 7.3 (100.0 ± 3.7) |
23.7 ± 4.0 ** (99.6 ± 2.2) |
34.4 ± 5.1 ** (102.7 ± 4.3) |
86.6 ± 2.6 ** (99.6 ± 2.4) |
103.4 ± 1.9 ** (92.5 ± 5.0) |
19.9 |
| NN-6 | 0.0 ± 6.0 (100.0 ± 4.5) |
−6.9 ± 13.7 (87.8 ± 4.7) |
−6.3 ± 12.0 (88.1 ± 1.0) |
77.4 ± 5.4 ** (90.8 ± 4.0) |
104.6 ± 2.1 ** (74.1 ± 3.3 #) |
54.1 |
| NN-7 | 0.0 ± 4.4 (100.0 ± 1.6) |
−9.5 ± 6.9 (94.7 ± 4.1) |
9.0 ± 7.6 (101.2 ± 2.2) |
79.4 ± 2.1 ** (102.3 ± 2.8) |
84.2 ± 4.2 ** (84.9 ± 0.9) |
46.6 |
| NN-8 | 0.0 ± 1.7 (100.0 ± 4.6) |
−3.3 ± 4.8 (100.8 ± 7.4) |
1.3 ± 7.9 (93.5 ± 3.2) |
57.6 ± 3.1 ** (95.6 ± 3.2) |
64.9 ± 2.3 ** (97.8 ± 3.8) |
78.9 |
Each value represents the mean ± S.E.M. (n = 4); asterisks denote significant differences from the control group, ** p < 0.01.; # cytotoxic effects were observed, and values in parentheses indicate cell viability (%) in MTT assay.
Figure 5.
Correlations between the melanogenesis inhibitory activities and total content. (a) Total content (%) of 10 alkaloids (1–10); (b) Total content (%) of two principal alkaloids (1 and 2). Total contents (%) of the alkaloids are presented in values reduced to the content of nuciferine (1), calculated based on the ratio of IC50 values (µg/mL) against melanogenesis inhibitory activities.